Association of human herpesvirus-6B with mesial temporal lobe epilepsy
- PMID: 17535102
- PMCID: PMC1880851
- DOI: 10.1371/journal.pmed.0040180
Association of human herpesvirus-6B with mesial temporal lobe epilepsy
Abstract
Background: Human herpesvirus-6 (HHV-6) is a beta-herpesvirus with 90% seroprevalence that infects and establishes latency in the central nervous system. Two HHV-6 variants are known: HHV-6A and HHV-6B. Active infection or reactivation of HHV-6 in the brain is associated with neurological disorders, including epilepsy, encephalitis, and multiple sclerosis. In a preliminary study, we found HHV-6B DNA in resected brain tissue from patients with mesial temporal lobe epilepsy (MTLE) and have localized viral antigen to glial fibrillary acidic protein (GFAP)-positive glia in the same brain sections. We sought, first, to determine the extent of HHV-6 infection in brain material resected from MTLE and non-MTLE patients; and second, to establish in vitro primary astrocyte cultures from freshly resected brain material and determine expression of glutamate transporters.
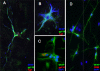
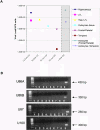
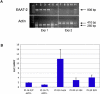
Methods and findings: HHV-6B infection in astrocytes and brain specimens was investigated in resected brain material from MTLE and non-MTLE patients using PCR and immunofluorescence. HHV-6B viral DNA was detected by TaqMan PCR in brain resections from 11 of 16 (69%) additional patients with MTLE and from zero of seven (0%) additional patients without MTLE. All brain regions that tested positive by HHV-6B variant-specific TaqMan PCR were positive for viral DNA by nested PCR. Primary astrocytes were isolated and cultured from seven epilepsy brain resections and astrocyte purity was defined by GFAP reactivity. HHV-6 gp116/54/64 antigen was detected in primary cultured GFAP-positive astrocytes from resected tissue that was HHV-6 DNA positive-the first demonstration of an ex vivo HHV-6-infected astrocyte culture isolated from HHV-6-positive brain material. Previous work has shown that MTLE is related to glutamate transporter dysfunction. We infected astrocyte cultures in vitro with HHV-6 and found a marked decrease in glutamate transporter EAAT-2 expression.
Conclusions: Overall, we have now detected HHV-6B in 15 of 24 patients with mesial temporal sclerosis/MTLE, in contrast to zero of 14 with other syndromes. Our results suggest a potential etiology and pathogenic mechanism for MTLE.
Conflict of interest statement
Figures

Similar articles
-
Pathogenic Role of Human Herpesvirus 6B Infection in Mesial Temporal Lobe Epilepsy.J Infect Dis. 2015 Oct 1;212(7):1014-21. doi: 10.1093/infdis/jiv160. Epub 2015 Apr 3. J Infect Dis. 2015. PMID: 25840441
-
Investigation of HSV-1, HSV-2, CMV, HHV-6 and HHV-8 DNA by real-time PCR in surgical resection materials of epilepsy patients with mesial temporal lobe sclerosis.J Neurol Sci. 2008 Jan 15;264(1-2):151-6. doi: 10.1016/j.jns.2007.08.010. Epub 2007 Sep 5. J Neurol Sci. 2008. PMID: 17804017
-
Differential HHV-6A gene expression in T cells and primary human astrocytes based on multi-virus array analysis.Glia. 2006 Jun;53(8):789-98. doi: 10.1002/glia.20333. Glia. 2006. PMID: 16541415
-
Molecular biology of human herpesviruses 6A and 6B.Infect Agents Dis. 1993 Dec;2(6):343-60. Infect Agents Dis. 1993. PMID: 8012736 Review.
-
HHV-6 and seizures.Herpes. 2005 Oct;12(2):46-9. Herpes. 2005. PMID: 16209861 Review.
Cited by
-
A Lesson from "The Brodie Ultimatum": The Locus of Control for Epilepsy is Outside the Therapeutic Alliance.Epilepsy Curr. 2013 Jan;13(1):17-9. doi: 10.5698/1535-7511-13.1.17. Epilepsy Curr. 2013. PMID: 23447731 Free PMC article. No abstract available.
-
Results from a hypothesis generating case-control study: herpes family viruses and schizophrenia among military personnel.Schizophr Bull. 2008 Nov;34(6):1182-8. doi: 10.1093/schbul/sbm139. Epub 2007 Dec 21. Schizophr Bull. 2008. PMID: 18156638 Free PMC article.
-
Human Herpesviruses 6A and 6B in Brain Diseases: Association versus Causation.Clin Microbiol Rev. 2020 Nov 11;34(1):e00143-20. doi: 10.1128/CMR.00143-20. Print 2020 Dec 16. Clin Microbiol Rev. 2020. PMID: 33177186 Free PMC article. Review.
-
Human herpesvirus 6 and 7 in febrile status epilepticus: the FEBSTAT study.Epilepsia. 2012 Sep;53(9):1481-8. doi: 10.1111/j.1528-1167.2012.03542.x. Epub 2012 Jun 14. Epilepsia. 2012. PMID: 22954016 Free PMC article.
-
Detection of human herpesvirus-6 variants in pediatric brain tumors: association of viral antigen in low grade gliomas.J Clin Virol. 2009 Sep;46(1):37-42. doi: 10.1016/j.jcv.2009.05.011. Epub 2009 Jun 7. J Clin Virol. 2009. PMID: 19505845 Free PMC article.
References
-
- Salahuddin SZ, Ablashi DV, Markham PD, Josephs SF, Sturzenegger S, et al. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986;234:596–601. - PubMed
-
- Kimberlin DW, Whitley RJ. Human herpesvirus-6: Neurologic implications of a newly-described viral pathogen. J Neurovirol. 1998;4:474–485. - PubMed
-
- Saito Y, Sharer LR, Dewhurst S, Blumberg BM, Hall CB, et al. Cellular localization of human herpesvirus-6 in the brains of children with AIDS encephalopathy. J Neurovirol. 1995;1:30–39. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

