All beta cells contribute equally to islet growth and maintenance
- PMID: 17535113
- PMCID: PMC1877817
- DOI: 10.1371/journal.pbio.0050163
All beta cells contribute equally to islet growth and maintenance
Abstract
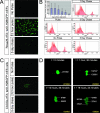
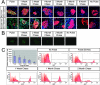
In healthy adult mice, the beta cell population is not maintained by stem cells but instead by the replication of differentiated beta cells. It is not known, however, whether all beta cells contribute equally to growth and maintenance, as it may be that some cells replicate while others do not. Understanding precisely which cells are responsible for beta cell replication will inform attempts to expand beta cells in vitro, a potential source for cell replacement therapy to treat diabetes. Two experiments were performed to address this issue. First, the level of fluorescence generated by a pulse of histone 2B-green fluorescent protein (H2BGFP) expression was followed over time to determine how this marker is diluted with cell division; a uniform loss of label across the entire beta cell population was observed. Second, clonal analysis of dividing beta cells was completed; all clones were of comparable size. These results support the conclusion that the beta cell pool is homogeneous with respect to replicative capacity and suggest that all beta cells are candidates for in vitro expansion. Given similar observations in the hepatocyte population, we speculate that for tissues lacking an adult stem cell, they are replenished equally by replication of all differentiated cells.
Conflict of interest statement
Figures




Comment in
-
All together now: pancreatic beta cells don't rely on a few to renew.PLoS Biol. 2007 Jul;5(7):e186. doi: 10.1371/journal.pbio.0050186. Epub 2007 Jun 12. PLoS Biol. 2007. PMID: 20076677 Free PMC article. No abstract available.
References
-
- Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–561. - PubMed
-
- Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. - PubMed
-
- Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. - PubMed
-
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. - PubMed
-
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

