Docosahexaenoic acid alters bilayer elastic properties
- PMID: 17535898
- PMCID: PMC1887599
- DOI: 10.1073/pnas.0701015104
Docosahexaenoic acid alters bilayer elastic properties
Abstract
At low micromolar concentrations, polyunsaturated fatty acids (PUFAs) alter the function of many membrane proteins. PUFAs exert their effects on unrelated proteins at similar concentrations, suggesting a common mode of action. Because lipid bilayers serve as the common "solvent" for membrane proteins, the common mechanism could be that PUFAs adsorb to the bilayer/solution interface to promote a negative-going change in lipid intrinsic curvature and, like other reversibly adsorbing amphiphiles, increase bilayer elasticity. PUFA adsorption thus would alter the bilayer deformation energy associated with protein conformational changes involving the protein/bilayer boundary, which would alter protein function. To explore the feasibility of such a mechanism, we used gramicidin (gA) analogues of different lengths together with bilayers of different thicknesses to assess whether docosahexaenoic acid (DHA) could exert its effects through a bilayer-mediated mechanism. Indeed, DHA increases gA channel appearance rates and lifetimes and decreases the free energy of channel formation. The appearance rate and lifetime changes increase with increasing channel-bilayer hydrophobic mismatch and are not related to differing DHA bilayer absorption coefficients. DHA thus alters bilayer elastic properties, not just lipid intrinsic curvature; the elasticity changes are important for DHA's bilayer-modifying actions. Oleic acid (OA), which has little effect on membrane protein function, exerts no such effects despite OA's adsorption coefficient being an order of magnitude greater than DHA's. These results suggest that DHA (and other PUFAs) may modulate membrane protein function by bilayer-mediated mechanisms that do not involve specific protein binding but rather changes in bilayer material properties.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


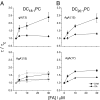
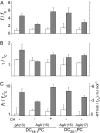
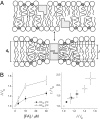
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

