Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance
- PMID: 17535918
- PMCID: PMC1887543
- DOI: 10.1073/pnas.0700117104
Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance
Abstract
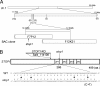
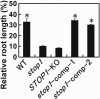
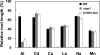
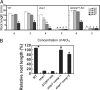
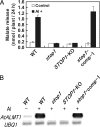
Acid soil syndrome causes severe yield losses in various crop plants because of the rhizotoxicities of ions, such as aluminum (Al(3+)). Although protons (H(+)) could be also major rhizotoxicants in some soil types, molecular mechanisms of their tolerance have not been identified yet. One mutant that was hypersensitive to H(+) rhizotoxicity was isolated from ethyl methanesulfonate mutagenized seeds, and a single recessive mutation was found on chromosome 1. Positional cloning followed by genomic sequence analysis revealed that a missense mutation in the zinc finger domain in a predicted Cys(2)His(2)-type zinc finger protein, namely sensitive to proton rhizotoxicity (STOP)1, is the cause of hypersensitivity to H(+) rhizotoxicity. The STOP1 protein belongs to a functionally unidentified subfamily of zinc finger proteins, which consists of two members in Arabidopsis based on a Blast search. The stop1 mutation resulted in no effects on cadmium, copper, lanthanum, manganese and sodium chloride sensitivitities, whereas it caused hypersensitivity to Al(3+) rhizotoxicity. This stop1 mutant lacked the induction of the AtALMT1 gene encoding a malate transporter, which is concomitant with Al-induced malate exudation. There was no induction of AtALMT1 by Al(3+) treatment in the stop1 mutant. These results indicate that STOP1 plays a critical role in Arabidopsis tolerance to major stress factors in acid soils.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Matsumoto H. Int Rev Cytol. 2000;200:1–46. - PubMed
-
- Ishitani M, Rao I, Wenzlb P, Beebea S, Tohme J. Field Crops Res. 2004;90:35–45.
-
- Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH. Ecol Appl. 1998;8:559–568.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

