The B''/PR72 subunit mediates Ca2+-dependent dephosphorylation of DARPP-32 by protein phosphatase 2A
- PMID: 17535922
- PMCID: PMC1887582
- DOI: 10.1073/pnas.0703589104
The B''/PR72 subunit mediates Ca2+-dependent dephosphorylation of DARPP-32 by protein phosphatase 2A
Abstract
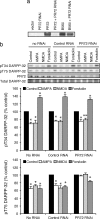
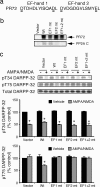
In dopaminoceptive neurons, dopamine- and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32) plays a central role in integrating the effects of dopamine and other neurotransmitters. Phosphorylation of DARPP-32 at Thr-34 by protein kinase A results in inhibition of protein phosphatase 1 (PP1), and phosphorylation at Thr-75 by Cdk5 (cyclin-dependent kinase 5) results in inhibition of protein kinase A. Dephosphorylation at Thr-34 involves primarily the Ca(2+)-dependent protein phosphatase, PP2B (calcineurin), whereas dephosphorylation of Thr-75 involves primarily PP2A, the latter being subject to control by both cAMP- and Ca(2+)-dependent regulatory mechanisms. In the present study, we have investigated the mechanism of Ca(2+)-dependent regulation of Thr-75 by PP2A. We show that the PR72 (or B'' or PPP2R3A) regulatory subunit of PP2A is highly expressed in striatum. Through the use of overexpression and down-regulation by using RNAi, we show that PP2A, in a heterotrimeric complex with the PR72 subunit, mediates Ca(2+)-dependent dephosphorylation at Thr-75 of DARPP-32. The PR72 subunit contains two Ca(2+) binding sites formed by E and F helices (EF-hands 1 and 2), and we show that the former is necessary for the ability of PP2A activity to be regulated by Ca(2+), both in vitro and in vivo. Our studies also indicate that the PR72-containing form of PP2A is necessary for the ability of glutamate acting at alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid and NMDA receptors to regulate Thr-75 dephosphorylation. These studies further our understanding of the complex signal transduction pathways that regulate DARPP-32. In addition, our studies reveal an alternative intracellular mechanism whereby Ca(2+) can activate serine/threonine phosphatase activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

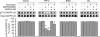
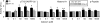
References
-
- Greengard P, Allen PB, Nairn AC. Neuron. 1999;23:435–447. - PubMed
-
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. Annu Rev Pharmacol Toxicol. 2004;44:269–296. - PubMed
-
- Fienberg AA, Hiroi N, Mermelstein PG, Song W, Snyder GL, Nishi A, Cheramy A, O'Callaghan JP, Miller DB, Cole DG, et al. Science. 1998;281:838–842. - PubMed
-
- Svenningsson P, Tzavara ET, Carruthers R, Rachleff I, Wattler S, Nehls M, McKinzie DL, Fienberg AA, Nomikos GG, Greengard P. Science. 2003;302:1412–1415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

