Block of CaV1.2 channels by Gd3+ reveals preopening transitions in the selectivity filter
- PMID: 17535959
- PMCID: PMC2151628
- DOI: 10.1085/jgp.200709733
Block of CaV1.2 channels by Gd3+ reveals preopening transitions in the selectivity filter
Abstract
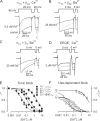
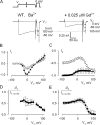
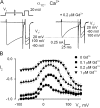
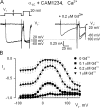
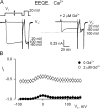
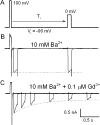
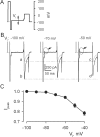
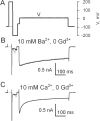
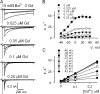
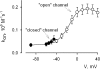
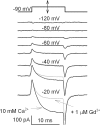
Using the lanthanide gadolinium (Gd(3+)) as a Ca(2+) replacing probe, we investigated the voltage dependence of pore blockage of Ca(V)1.2 channels. Gd(+3) reduces peak currents (tonic block) and accelerates decay of ionic current during depolarization (use-dependent block). Because diffusion of Gd(3+) at concentrations used (<1 microM) is much slower than activation of the channel, the tonic effect is likely to be due to the blockage that occurred in closed channels before depolarization. We found that the dose-response curves for the two blocking effects of Gd(3+) shifted in parallel for Ba(2+), Sr(2+), and Ca(2+) currents through the wild-type channel, and for Ca(2+) currents through the selectivity filter mutation EEQE that lowers the blocking potency of Gd(3+). The correlation indicates that Gd(3+) binding to the same site causes both tonic and use-dependent blocking effects. The apparent on-rate for the tonic block increases with the prepulse voltage in the range -60 to -45 mV, where significant gating current but no ionic current occurs. When plotted together against voltage, the on-rates of tonic block (-100 to -45 mV) and of use-dependent block (-40 to 40 mV) fall on a single sigmoid that parallels the voltage dependence of the gating charge. The on-rate of tonic block by Gd(3+) decreases with concentration of Ba(2+), indicating that the apparent affinity of the site to permeant ions is about 1 mM in closed channels. Therefore, we propose that at submicromolar concentrations, Gd(3+) binds at the entry to the selectivity locus and that the affinity of the site for permeant ions decreases during preopening transitions of the channel.
Figures



Comment in
-
And yet it moves: conformational States of the Ca2+ channel pore.J Gen Physiol. 2007 Jun;129(6):457-9. doi: 10.1085/jgp.200709814. J Gen Physiol. 2007. PMID: 17535958 Free PMC article. No abstract available.
References
-
- Armstrong, C.M., and J. Neyton. 1991. Ion permeation through calcium channels. A one site model. Ann. NY Acad. Sci. 635:18–25. - PubMed
-
- Bahinski, A., A. Yatani, G. Mikala, S. Tang, S. Yamamoto, and A. Schwartz. 1997. Charged amino acids near the pore entrance influence ion-conduction of a human L-type cardiac calcium channel. Mol. Cell. Biochem. 166:125–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

