The syndecan-1 heparan sulfate proteoglycan is a viable target for myeloma therapy
- PMID: 17536013
- PMCID: PMC1976367
- DOI: 10.1182/blood-2007-04-082495
The syndecan-1 heparan sulfate proteoglycan is a viable target for myeloma therapy
Abstract
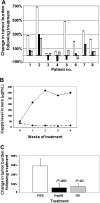
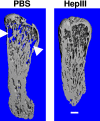
The heparan sulfate proteoglycan syndecan-1 is expressed by myeloma cells and shed into the myeloma microenvironment. High levels of shed syndecan-1 in myeloma patient sera correlate with poor prognosis and studies in animal models indicate that shed syndecan-1 is a potent stimulator of myeloma tumor growth and metastasis. Overexpression of extracellular endosulfatases, enzymes which remove 6-O sulfate groups from heparan sulfate chains, diminishes myeloma tumor growth in vivo. Together, these findings identify syndecan-1 as a potential target for myeloma therapy. Here, 3 different strategies were tested in animal models of myeloma with the following results: (1) treatment with bacterial heparinase III, an enzyme that degrades heparan sulfate chains, dramatically inhibited the growth of primary tumors in the human severe combined immunodeficient (SCID-hu) model of myeloma; (2) treatment with an inhibitor of human heparanase, an enzyme that synergizes with syndecan-1 in promoting myeloma progression, blocked the growth of myeloma in vivo; and (3) knockdown of syndecan-1 expression by RNAi diminished and delayed myeloma tumor development in vivo. These results confirm the importance of syndecan-1 in myeloma pathobiology and provide strong evidence that disruption of the normal function or amount of syndecan-1 or its heparan sulfate chains is a valid therapeutic approach for this cancer.
Figures

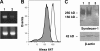

References
-
- Ridley RC, Xiao HQ, Hata H, Woodliff J, Epstein J, Sanderson RD. Expression of syndecan regulates human myeloma plasma cell adhesion to type I collagen. Blood. 1993;81:767–774. - PubMed
-
- Wijdenes J, Vooijs WC, Clement C, et al. A plasmocyte selective monoclonal antibody (B-B4) recognizes syndecan-1. Br J Haematol. 1996;94:318–323. - PubMed
-
- Bayer-Garner IB, Sanderson RD, Dhodapkar MV, Owens RB, Wilson CS. Syndecan-1 (CD138) immunoreactivity in bone marrow biopsies of multiple myeloma: shed syndecan-1 accumulates in fibrotic regions. Mod Pathol. 2001;14:1052–1058. - PubMed
-
- Dhodapkar MV, Kelly T, Theus A, Athota AB, Barlogie B, Sanderson RD. Elevated levels of shed syndecan-1 correlate with tumour mass and decreased matrix metalloproteinase-9 activity in the serum of patients with multiple myeloma. Br J Haematol. 1997;99:368–371. - PubMed
-
- Seidel C, Sundan A, Hjorth M, et al. Serum syndecan-1: a new independent prognostic marker in multiple myeloma. Blood. 2000;95:388–392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

