Understanding how the thiolate sulfur contributes to the function of the non-heme iron enzyme superoxide reductase
- PMID: 17536780
- PMCID: PMC3703784
- DOI: 10.1021/ar600059h
Understanding how the thiolate sulfur contributes to the function of the non-heme iron enzyme superoxide reductase
Abstract
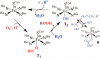
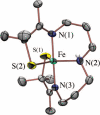
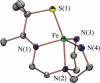
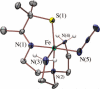
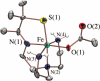
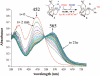
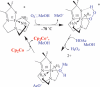
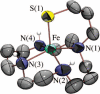
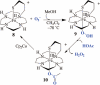
Toxic superoxide radicals, generated via adventitious reduction of dioxygen, have been implicated in a number of disease states. The cysteinate-ligated non-heme iron enzyme superoxide reductase (SOR) degrades superoxide via reduction. Biomimetic analogues which provide insight into why nature utilizes a trans-thiolate to promote SOR function are described. Spectroscopic and/or structural characterization of the first examples of thiolate-ligated Fe (III)-peroxo complexes provides important benchmark parameters for the identification of biological intermediates. Oxidative addition of superoxide is favored by low redox potentials. The trans influence of the thiolate appears to significantly weaken the Fe-O peroxo bond, favoring proton-induced release of H 2O 2 from a high-spin Fe(III)-OOH complex.
Figures
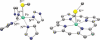
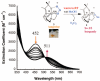
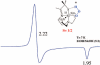
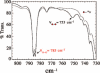
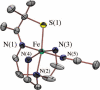
References
-
- Costas M, Mehn MP, Jensen MP, Que LJ. Dioxygen Activation at Mononuclear Nonheme Iron Active Sites: Enzymes, Models, and Intermediates. Chem. Rev. 2004;104:939–986. - PubMed
-
- Kovacs JA. How Iron Activates O2. Science. 2003;299:1024–1025. - PubMed
-
- Hausinger RP. Fe(II) α-Ketoglutarate-Dependent Hydroxylases and Related Enzymes. Crit. Rev. Biochem. Mol. Biol. 2004;39:1–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

