Calcicludine binding to the outer pore of L-type calcium channels is allosterically coupled to dihydropyridine binding
- PMID: 17536837
- PMCID: PMC2526233
- DOI: 10.1021/bi7001696
Calcicludine binding to the outer pore of L-type calcium channels is allosterically coupled to dihydropyridine binding
Abstract
How dihydropyridines modulate L-type voltage-gated Ca2+ channels is not known. Dihydropyridines bind cooperatively with Ca2+ binding to the selectivity filter, suggesting that they alter channel activity by promoting structural rearrangements in the pore. We used radioligand binding and patch-clamp electrophysiology to demonstrate that calcicludine, a toxin from the venom of the green mamba snake, binds in the outer vestibule of the pore and, like Ca2+, is a positive modulator of dihydropyridine binding. Data were fit using an allosteric scheme where dissociation constants for dihydropyridine and calcicludine binding, KDHP and KCaC, are linked via the coupling factor, alpha. Nine acidic amino acids located within the S5-Pore-helix segment of repeat III were sequentially changed to alanine in groups of three, resulting in the mutant channels, Mut-A, Mut-B, and Mut-C. Mut-A, whose substitutions are proximal to IIIS5, exhibits a 4.5-fold reduction in dihydropyridine binding and is insensitive to calcicludine binding. Block of Mut-A currents by calcicludine is indistinguishable from wild-type, indicating that KCaC is unchanged and that the coupling between dihydropyridine and calcicludine binding (i.e., alpha) is disrupted. Mut-B and Mut-C possess KDHP values that resemble that of the wild type. Mut-C, the most C-terminal of the mutant channels, is insensitive to calcicludine binding and block. KCaC values for the Mut-C single mutants, E1122A, D1127A, and D1129A, increase from 0.3 (wild type) to 1.14, 2.00, and 20.5 microM, respectively. Together, these findings suggest that dihydropyridine antagonist and calcicludine binding to L-type Ca2+ channels promote similar structural changes in the pore that stabilize the channel in a nonconducting, blocked state.
Figures

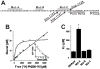
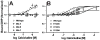

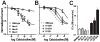

References
-
- Hille B. Ion channels of excitable membranes. 3. Sinaur Associates, Inc.; Sunderland, MA: 2001.
-
- Herbert LG. Membrane pathways for drug/ion channel interactions: molecular basis for pharmacokinetic properties. Drug Development and Research. 1994:214–224.
-
- Bangalore R, Baindur N, Rutledge A, Triggle DJ, Kass RS. L-type calcium channels: asymmetrical intramembrane binding domain revealed by variable length, permanently charged 1,4-dihydropyridines. Mol Pharmacol. 1994:660–666. - PubMed
-
- He M, Bodi I, Mikala G, Schwartz A. Motif III S5 of L-type calcium channels is involved in the dihydropyridine binding site. A combined radioligand binding and electrophysiological study. J Biol Chem. 1997;272:2629–2633. - PubMed
-
- Mitterdorfer J, Wang Z, Sinnegger MJ, Hering S, Striessnig J, Grabner M, Glossmann H. Two amino acid residues in the IIIS5 segment of L-type calcium channels differentially contribute to 1,4-dihydropyridine sensitivity. J Biol Chem. 1996:30330–30335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

