Effects of tension-compression nonlinearity on solute transport in charged hydrated fibrous tissues under dynamic unconfined compression
- PMID: 17536910
- PMCID: PMC2671022
- DOI: 10.1115/1.2720920
Effects of tension-compression nonlinearity on solute transport in charged hydrated fibrous tissues under dynamic unconfined compression
Abstract
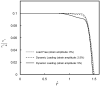
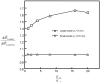
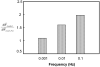
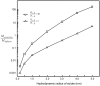
Cartilage is a charged hydrated fibrous tissue exhibiting a high degree of tension-compression nonlinearity (i.e., tissue anisotropy). The effect of tension-compression nonlinearity on solute transport has not been investigated in cartilaginous tissue under dynamic loading conditions. In this study, a new model was developed based on the mechano-electrochemical mixture model [Yao and Gu, 2007, J. Biomech. Model Mechanobiol., 6, pp. 63-72, Lai et al., 1991, J. Biomech. Eng., 113, pp. 245-258], and conewise linear elasticity model [Soltz and Ateshian, 2000, J. Biomech. Eng., 122, pp. 576-586; Curnier et al., 1995, J. Elasticity, 37, pp. 1-38]. The solute desorption in cartilage under unconfined dynamic compression was investigated numerically using this new model. Analyses and results demonstrated that a high degree of tissue tension-compression nonlinearity could enhance the transport of large solutes considerably in the cartilage sample under dynamic unconfined compression, whereas it had little effect on the transport of small solutes (at 5% dynamic strain level). The loading-induced convection is an important mechanism for enhancing the transport of large solutes in the cartilage sample with tension-compression nonlinearity. The dynamic compression also promoted diffusion of large solutes in both tissues with and without tension-compression nonlinearity. These findings provide a new insight into the mechanisms of solute transport in hydrated, fibrous soft tissues.
Figures



Similar articles
-
Physical signals and solute transport in cartilage under dynamic unconfined compression: finite element analysis.Ann Biomed Eng. 2004 Mar;32(3):380-90. doi: 10.1023/b:abme.0000017540.84764.6f. Ann Biomed Eng. 2004. PMID: 15095812
-
Experimental verification of the roles of intrinsic matrix viscoelasticity and tension-compression nonlinearity in the biphasic response of cartilage.J Biomech Eng. 2003 Feb;125(1):84-93. doi: 10.1115/1.1531656. J Biomech Eng. 2003. PMID: 12661200
-
The role of flow-independent viscoelasticity in the biphasic tensile and compressive responses of articular cartilage.J Biomech Eng. 2001 Oct;123(5):410-7. doi: 10.1115/1.1392316. J Biomech Eng. 2001. PMID: 11601725
-
Modeling of neutral solute transport in a dynamically loaded porous permeable gel: implications for articular cartilage biosynthesis and tissue engineering.J Biomech Eng. 2003 Oct;125(5):602-14. doi: 10.1115/1.1611512. J Biomech Eng. 2003. PMID: 14618919 Free PMC article.
-
Mechano-electrochemical properties of articular cartilage: their inhomogeneities and anisotropies.Annu Rev Biomed Eng. 2002;4:175-209. doi: 10.1146/annurev.bioeng.4.110701.120309. Epub 2002 Mar 22. Annu Rev Biomed Eng. 2002. PMID: 12117756 Review.
Cited by
-
Structural biomechanics modulate intramuscular distribution of locally delivered drugs.J Biomech. 2008 Sep 18;41(13):2884-91. doi: 10.1016/j.jbiomech.2008.06.025. Epub 2008 Aug 15. J Biomech. 2008. PMID: 18706562 Free PMC article.
-
Effects of mechanical compression on metabolism and distribution of oxygen and lactate in intervertebral disc.J Biomech. 2008;41(6):1184-96. doi: 10.1016/j.jbiomech.2008.02.002. J Biomech. 2008. PMID: 18374341 Free PMC article.
-
Cell viability in intervertebral disc under various nutritional and dynamic loading conditions: 3d finite element analysis.J Biomech. 2012 Nov 15;45(16):2769-77. doi: 10.1016/j.jbiomech.2012.08.044. Epub 2012 Oct 4. J Biomech. 2012. PMID: 23040882 Free PMC article.
-
Effects of diurnal loading on the transport of charged antibiotics into intervertebral discs.J Biomech. 2019 Apr 18;87:177-182. doi: 10.1016/j.jbiomech.2019.03.013. Epub 2019 Mar 18. J Biomech. 2019. PMID: 30905406 Free PMC article.
-
Three-dimensional inhomogeneous triphasic finite-element analysis of physical signals and solute transport in human intervertebral disc under axial compression.J Biomech. 2007;40(9):2071-7. doi: 10.1016/j.jbiomech.2006.10.001. Epub 2006 Nov 22. J Biomech. 2007. PMID: 17125776 Free PMC article.
References
-
- Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic Creep and Stress Relaxation of Articular Cartilage in Compression: Theory and Experiments. J. Biomech. Eng. 1980;102:73–84. - PubMed
-
- Soltz MA, Ateshian GA. Experimental Verification and Theoretical Prediction of Cartilage Interstitial Fluid Pressurization at An Impermeable Contact Interface in Confined Compression. J. Biomech. 1998;31(10):927–934. - PubMed
-
- Ohshima H, Urban JP. The Effect of Lactate and pH on Proteoglycan and Protein Synthesis Rates in the Intervertebral Disc. Spine. 1992;17(9):1079–1082. - PubMed
-
- Ysart GE, Mason RM. Responses of Articular Cartilage Explant Cultures to Different Oxygen Tensions. Biochim. Biophys. Acta. 1994;1221(1):15–20. - PubMed
-
- Ishihara H, Urban JP. Effects of Low Oxygen Concentrations and Metabolic Inhibitors on Proteoglycan and Protein Synthesis Rates in the Intervertebral disc. J. Orthop. Res. 1999;17(6):829–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

