Front-line bevacizumab in combination with oxaliplatin, leucovorin and 5-fluorouracil (FOLFOX) in patients with metastatic colorectal cancer: a multicenter phase II study
- PMID: 17537235
- PMCID: PMC1894803
- DOI: 10.1186/1471-2407-7-91
Front-line bevacizumab in combination with oxaliplatin, leucovorin and 5-fluorouracil (FOLFOX) in patients with metastatic colorectal cancer: a multicenter phase II study
Abstract
Purpose: To evaluate the efficacy and the toxicity of front line FOLFOX4 combined with bevacizumab in patients with metastatsic CRC (mCRC).
Patients and methods: Chemotherapy-naïve patients with mCRC, received bevacizumab (5 mg/kg every 2 weeks d1), oxaliplatin (85 mg/m2 on d1), leucovorin (200 mg/m2) on days 1 and 2 and 5-Fluorouracil (400 mg/m2 as i.v. bolus and 600 mg/m2 as 22 h i.v. continuous infusion on days 1 and 2) every 2 weeks.
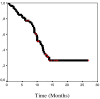
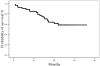
Results: Fifty three patients (46 with a PS 0-1) were enrolled. Complete and partial response was achieved in eight (15.1%) and 28 (52.8%) patients, respectively (ORR: 67.9%; 95% C.I.: 53.8%-92%); 11 (20.7%) patients had stable disease and six (11.3%) progressive disease. With a median follow up period of 13.5 months, time to tumor progression was 11 months while the median survival has not yet been reached; the probability of 1-, 2- and 3- year survival was 79.8%, 63.8% and 58.3%, respectively; Two patients relapsed during the follow up period. Eight (15%) patients underwent metastasectomy with R0 resections. Grade 3-4 neutropenia occurred in 15.1% of patients and one (1.9%) of them presented febrile neutropenia. Non-hematologic toxicity included grade 3 diarrhea (7.6%) and grade 2 and 3 neurotoxicity in 16.9 and 15.1% of patients, respectively. One (1.9%) patient presented pulmonary embolism and one (1.9%) cardiac ischaemia. There was one (1.9%) sudden death after the first cycle.
Conclusion: The combination of FOLFOX4/bevacizumab appears to be highly effective, well tolerated and merits further evaluation in patients with mCRC.
Figures
References
-
- Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. - PubMed
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/S0140-6736(00)02034-1. - DOI - PubMed
-
- Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de GA. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical