In vitro binding and survival assays of Leishmania parasites to peripherical blood monocytes and monocyte-derived macrophages isolated from dogs naturally and experimentally infected with Leishmania (Leishmania) chagasi
- PMID: 17537246
- PMCID: PMC1894629
- DOI: 10.1186/1746-6148-3-11
In vitro binding and survival assays of Leishmania parasites to peripherical blood monocytes and monocyte-derived macrophages isolated from dogs naturally and experimentally infected with Leishmania (Leishmania) chagasi
Abstract
Background: There are a few works considering the characterization of canine monocyte-derived macrophages as well as a standardized procedure for isolation, culture, and infection of these cells with Leishmania. We have performed several modifications in order to improve the canine monocyte-derived macrophage cultures. In addition, we have done a comparative study between monocytes and monocyte-derived macrophages from dogs naturally and experimentally infected with L. chagasi.
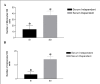
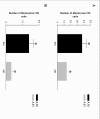
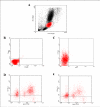
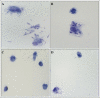
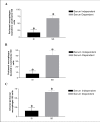
Results: In the presence of exogenous serum, opsonized Leishmania promastigotes binds better to monocytes/macrophages than without serum. Otherwise, this binding occurs due to the strict correlation between the opsonized biologic particles with the third receptor of the complement (CR3-CD11b/CD18). In fact, our assays with CD11b confirmed the importance of this receptor for canine cells and the L. chagasi experimental system. Moreover, monocytes obtained from naturally infected dogs have shown a higher number of monocytes bounded to promastigotes. The experimental results regarding survival have shown that promastigote forms of opsonized L. chagasi were more infective, because we found higher numbers of promastigotes bound to the different cells. As a consequence, after forty-eight hours of binding, higher numbers of amastigotes appeared inside monocyte-macrophages.
Conclusion: These studies have given support to continue comparative studies involving canine monocytes, monocyte-derived macrophages and peritoneal macrophages. Since we have standardized the canine cell culture, we are looking forward to determining the phenotypic properties of these cells before and after L. chagasi infection using flow cytometry.
Figures

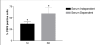
References
-
- Deane LM, Deane MP. Visceral leishmaniasis in Brazil: geographical distribution and trnsmission. Rev Inst Med Trop Sao Paulo. 1962;4:198–212. - PubMed
-
- Pinelli E, Rutten VP, Bruysters M, Moore PF, Ruitenberg EJ. Compensation for decreased expression of B7 molecules on Leishmania infantum-infected canine macrophages results in restoration of parasite-specific T-cell proliferation and gamma interferon production. Infect Immun. 1999;67:237–243. - PMC - PubMed
-
- Shaw SE, Anderson NV. Isolation and functional analysis of normal canine blood monocytes and resident alveolar macrophages. Am J Vet Res. 1984;45:87–90. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

