Protein kinase Cepsilon makes the life and death decision
- PMID: 17537614
- PMCID: PMC1986651
- DOI: 10.1016/j.cellsig.2007.04.008
Protein kinase Cepsilon makes the life and death decision
Abstract
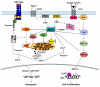
Cancer is caused by dysregulation in cellular signaling systems that control cell proliferation, differentiation and cell death. Protein kinase C (PKC), a family of serine/threonine kinases, plays an important role in the growth factor signal transduction pathway. PKCepsilon, however, is the only PKCepsilon isozyme that has been considered as an oncogene. It can contribute to malignancy by enhancing cell proliferation or by inhibiting cell death. This review focuses on how PKCepsilon collaborates with other signaling pathways, such as Ras/Raf/ERK and Akt, to regulate cell survival and cell death. We have also discussed how PKCepsilon mediates its antiapoptotic signal by altering the level or function of pro- and antiapoptotic Bcl-2 family members.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

