Nuclear relocation of the nephrin and CD2AP-binding protein dendrin promotes apoptosis of podocytes
- PMID: 17537921
- PMCID: PMC1891229
- DOI: 10.1073/pnas.0700917104
Nuclear relocation of the nephrin and CD2AP-binding protein dendrin promotes apoptosis of podocytes
Abstract
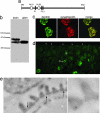
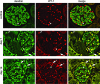
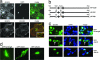
Kidney podocytes and their slit diaphragms (SDs) form the final barrier to urinary protein loss. There is mounting evidence that SD proteins also participate in intracellular signaling pathways. The SD protein nephrin serves as a component of a signaling complex that directly links podocyte junctional integrity to actin cytoskeletal dynamics. Another SD protein, CD2-associated protein (CD2AP), is an adaptor molecule involved in podocyte homeostasis that can repress proapoptotic TGF-beta signaling in podocytes. Here we show that dendrin, a protein originally identified in telencephalic dendrites, is a constituent of the SD complex, where it directly binds to nephrin and CD2AP. In experimental glomerulonephritis, dendrin relocates from the SD to the nucleus of injured podocytes. High-dose, proapoptotic TGF-beta1 directly promotes the nuclear import of dendrin, and nuclear dendrin enhances both staurosporine- and TGF-beta1-mediated apoptosis. In summary, our results identify dendrin as an SD protein with proapoptotic signaling properties that accumulates in the podocyte nucleus in response to glomerular injury and provides a molecular target to tackle proteinuric kidney diseases. Nuclear relocation of dendrin may provide a mechanism whereby changes in SD integrity could translate into alterations of podocyte survival under pathological conditions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

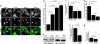
References
-
- Somlo S, Mundel P. Nat Genet. 2000;24:333–335. - PubMed
-
- Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, et al. Mol Cell. 1998;1:575–582. - PubMed
-
- Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS. Science. 1999;286:312–315. - PubMed
-
- Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodriguez-Perez JC, Allen PG, Beggs AH, Pollak MR. Nat Genet. 2000;24:251–256. - PubMed
-
- Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C. Nat Genet. 2000;24:349–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

