The clathrin adaptor complex AP-1 binds HIV-1 and MLV Gag and facilitates their budding
- PMID: 17538020
- PMCID: PMC1949356
- DOI: 10.1091/mbc.e06-12-1147
The clathrin adaptor complex AP-1 binds HIV-1 and MLV Gag and facilitates their budding
Abstract
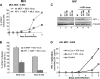
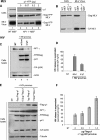
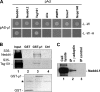
Retroviral assembly is driven by Gag, and nascent viral particles escape cells by recruiting the machinery that forms intralumenal vesicles of multivesicular bodies. In this study, we show that the clathrin adaptor complex AP-1 is involved in retroviral release. The absence of AP-1mu obtained by genetic knock-out or by RNA interference reduces budding of murine leukemia virus (MLV) and HIV-1, leading to a delay of viral propagation in cell culture. In contrast, overexpression of AP-1mu enhances release of HIV-1 Gag. We show that the AP-1 complex facilitates retroviral budding through a direct interaction between the matrix and AP-1mu. Less MLV Gag is found associated with late endosomes in cells lacking AP-1, and our results suggest that AP-1 and AP-3 could function on the same pathway that leads to Gag release. In addition, we find that AP-1 interacts with Tsg101 and Nedd4.1, two cellular proteins known to be involved in HIV-1 and MLV budding. We propose that AP-1 promotes Gag release by transporting it to intracellular sites of active budding, and/or by facilitating its interactions with other cellular partners.
Figures





References
-
- Babst M., Katzmann D. J., Estepa-Sabal E. J., Meerloo T., Emr S. D. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell. 2002a;3:271–282. - PubMed
-
- Babst M., Katzmann D. J., Snyder W. B., Wendland B., Emr S. D. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell. 2002b;3:283–289. - PubMed
-
- Basyuk E., Galli T., Mougel M., Blanchard J., Sitbon M., Bertrand E. Retroviral genomic RNAs are transported to the plasma membrane by endosomal vesicles. Dev. Cell. 2003;5:161–174. - PubMed
-
- Batonick M., Favre M., Boge M., Spearman P., Honing S., Thali M. Interaction of HIV-1 Gag with the clathrin-associated adaptor AP-2. Virology. 2005;342:190–200. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

