Mechanism underlying the iron-dependent nuclear export of the iron-responsive transcription factor Aft1p in Saccharomyces cerevisiae
- PMID: 17538022
- PMCID: PMC1949351
- DOI: 10.1091/mbc.e06-11-1054
Mechanism underlying the iron-dependent nuclear export of the iron-responsive transcription factor Aft1p in Saccharomyces cerevisiae
Abstract
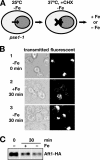
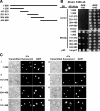
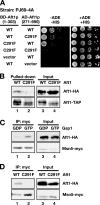
Aft1p is an iron-responsive transcriptional activator that plays a central role in maintaining iron homeostasis in Saccharomyces cerevisiae. Aft1p is regulated primarily by iron-induced shuttling of the protein between the nucleus and cytoplasm, but its nuclear import is not regulated by iron. Here, we have shown that the nuclear export of Aft1p is promoted in the presence of iron and that Msn5p is the nuclear export receptor (exportin) for Aft1p. Msn5p recognizes Aft1p in the iron-replete condition. Phosphorylation of S210 and S224 in Aft1p, which is not iron dependent, and the iron-induced intermolecular interaction of Aft1p are both essential for its recognition by Msn5p. Mutation of Cys291 of Aft1p to Phe, which causes Aft1p to be retained in the nucleus and results in constitutive activation of Aft1-target genes, disrupts the intermolecular interaction of Aft1p. Collectively, these results suggest that iron induces a conformational change in Aft1p, in which Aft1p Cys291 plays a critical role, and that, in turn, Aft1p is recognized by Msn5p and exported into the cytoplasm in an iron-dependent manner.
Figures




Similar articles
-
Pse1p mediates the nuclear import of the iron-responsive transcription factor Aft1p in Saccharomyces cerevisiae.J Biol Chem. 2003 Dec 12;278(50):50120-7. doi: 10.1074/jbc.M305046200. Epub 2003 Oct 1. J Biol Chem. 2003. PMID: 14523005
-
Iron-induced dissociation of the Aft1p transcriptional regulator from target gene promoters is an initial event in iron-dependent gene suppression.Mol Cell Biol. 2012 Dec;32(24):4998-5008. doi: 10.1128/MCB.00726-12. Epub 2012 Oct 8. Mol Cell Biol. 2012. PMID: 23045394 Free PMC article.
-
The Hog1p kinase regulates Aft1p transcription factor to control iron accumulation.Biochim Biophys Acta Mol Cell Biol Lipids. 2018 Jan;1863(1):61-70. doi: 10.1016/j.bbalip.2017.10.001. Epub 2017 Oct 12. Biochim Biophys Acta Mol Cell Biol Lipids. 2018. PMID: 29032057
-
The ins and outs of nuclear re-export of retrogradely transported tRNAs in Saccharomyces cerevisiae.Nucleus. 2010 May-Jun;1(3):224-30. doi: 10.4161/nucl.1.3.11250. Epub 2010 Jan 13. Nucleus. 2010. PMID: 21327067 Free PMC article. Review.
-
Signaling pathways governing iron homeostasis in budding yeast.Mol Microbiol. 2018 Aug;109(4):422-432. doi: 10.1111/mmi.14009. Epub 2018 Sep 4. Mol Microbiol. 2018. PMID: 29995317 Review.
Cited by
-
Monothiol CGFS glutaredoxins and BolA-like proteins: [2Fe-2S] binding partners in iron homeostasis.Biochemistry. 2012 Jun 5;51(22):4377-89. doi: 10.1021/bi300393z. Epub 2012 May 23. Biochemistry. 2012. PMID: 22583368 Free PMC article. Review.
-
An Internal Promoter Drives the Expression of a Truncated Form of CCC1 Capable of Protecting Yeast from Iron Toxicity.Microorganisms. 2021 Jun 20;9(6):1337. doi: 10.3390/microorganisms9061337. Microorganisms. 2021. PMID: 34203091 Free PMC article.
-
Aft1 Nuclear Localization and Transcriptional Response to Iron Starvation Rely upon TORC2/Ypk1 Signaling and Sphingolipid Biosynthesis.Int J Mol Sci. 2023 Jan 26;24(3):2438. doi: 10.3390/ijms24032438. Int J Mol Sci. 2023. PMID: 36768760 Free PMC article.
-
Iron sensing and regulation in Saccharomyces cerevisiae: Ironing out the mechanistic details.Curr Opin Microbiol. 2013 Dec;16(6):662-8. doi: 10.1016/j.mib.2013.07.020. Epub 2013 Aug 17. Curr Opin Microbiol. 2013. PMID: 23962819 Free PMC article. Review.
-
A chemical potentiator of copper-accumulation used to investigate the iron-regulons of Saccharomyces cerevisiae.Mol Microbiol. 2014 Jul;93(2):317-30. doi: 10.1111/mmi.12661. Epub 2014 Jun 15. Mol Microbiol. 2014. PMID: 24895027 Free PMC article.
References
-
- Askwith C. C., de Silva D., Kaplan J. Molecular biology of iron acquisition in Saccharomyces cerevisiae. Mol. Microbiol. 1996;20:27–34. - PubMed
-
- Askwith C., Eide D., Van Ho A., Bernard P. S., Li L., Davis-Kaplan S., Sipe D. M., Kaplan J. The FET3 gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell. 1994;76:403–410. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

