Identification of Arabidopsis cyclase-associated protein 1 as the first nucleotide exchange factor for plant actin
- PMID: 17538023
- PMCID: PMC1949352
- DOI: 10.1091/mbc.e06-11-1041
Identification of Arabidopsis cyclase-associated protein 1 as the first nucleotide exchange factor for plant actin
Abstract
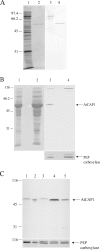
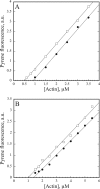
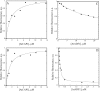
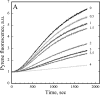
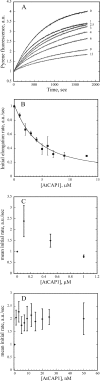
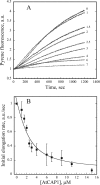
The actin cytoskeleton powers organelle movements, orchestrates responses to abiotic stresses, and generates an amazing array of cell shapes. Underpinning these diverse functions of the actin cytoskeleton are several dozen accessory proteins that coordinate actin filament dynamics and construct higher-order assemblies. Many actin-binding proteins from the plant kingdom have been characterized and their function is often surprisingly distinct from mammalian and fungal counterparts. The adenylyl cyclase-associated protein (CAP) has recently been shown to be an important regulator of actin dynamics in vivo and in vitro. The disruption of actin organization in cap mutant plants indicates defects in actin dynamics or the regulated assembly and disassembly of actin subunits into filaments. Current models for actin dynamics maintain that actin-depolymerizing factor (ADF)/cofilin removes ADP-actin subunits from filament ends and that profilin recharges these monomers with ATP by enhancing nucleotide exchange and delivery of subunits onto filament barbed ends. Plant profilins, however, lack the essential ability to stimulate nucleotide exchange on actin, suggesting that there might be a missing link yet to be discovered from plants. Here, we show that Arabidopsis thaliana CAP1 (AtCAP1) is an abundant cytoplasmic protein; it is present at a 1:3 M ratio with total actin in suspension cells. AtCAP1 has equivalent affinities for ADP- and ATP-monomeric actin (Kd approximately 1.3 microM). Binding of AtCAP1 to ATP-actin monomers inhibits polymerization, consistent with AtCAP1 being an actin sequestering protein. However, we demonstrate that AtCAP1 is the first plant protein to increase the rate of nucleotide exchange on actin. Even in the presence of ADF/cofilin, AtCAP1 can recharge actin monomers and presumably provide a polymerizable pool of subunits to profilin for addition onto filament ends. In turnover assays, plant profilin, ADF, and CAP act cooperatively to promote flux of subunits through actin filament barbed ends. Collectively, these results and our understanding of other actin-binding proteins implicate CAP1 as a central player in regulating the pool of unpolymerized ATP-actin.
Figures


References
-
- Alonso J. M., et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. - PubMed
-
- Andrianantoandro E., Pollard T. D. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol. Cell. 2006;24:13–23. - PubMed
-
- Balcer H. I., Goodman A. L., Rodal A. A., Smith E., Kugler J., Heuser J. E., Goode B. L. Coordinated regulation of actin filament turnover by a high-molecular-weight Srv2/CAP complex, cofilin, profilin, and Aip1. Curr. Biol. 2003;13:2159–2169. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

