Potentiometric and relaxometric properties of a gadolinium-based MRI contrast agent for sensing tissue pH
- PMID: 17539632
- PMCID: PMC2533746
- DOI: 10.1021/ic0702926
Potentiometric and relaxometric properties of a gadolinium-based MRI contrast agent for sensing tissue pH
Abstract
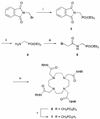
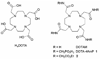
The pH-sensitive contrast agent, GdDOTA-4AmP (Gd1) has been successfully used to map tissue pH by MRI. Further studies now demonstrate that two distinct chemical forms of the complex can be prepared depending upon the pH at which Gd(3+) is mixed with ligand 1. The desired pH-sensitive form of this complex, referred to here as a Type II complex, is obtained as the exclusive product only when the complexation reaction is performed above pH 8. At lower pH values, a second complex is formed that, by analogy with an intermediate formed during the preparation of GdDOTA, we tentatively assign to a Type I complex where the Gd(3+) is coordinated only by the appended side-chain arms of 1. The proportion of Type I complex formed is largely determined by the pH of the complexation reaction. The magnitude of the pH-dependent change in the relaxivity of Gd1 was found to be less than earlier reported (Zhang, S.; Wu, K.; Sherry, A. D. Angew. Chem., Int. Ed. 1999, 38, 3192), likely due to contamination of the earlier sample by an unknown amount of Type I complex. Examination of the nuclear magnetic relaxation dispersion and relaxivity temperature profiles, coupled with information from potentiometric titrations, shows that the amphoteric character of the phosphonate side chains enables rapid prototropic exchange between the single bound water of the complex with the bulk water thereby giving Gd1 a unique pH-dependent relaxivity that is quite useful for the pH mapping of tissues by MRI.
Figures







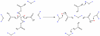
References
-
- Sennoune Souad R, Luo D, Martinez-Zaguilan R. Cell Biochem. Biophys. 2004;40:185. - PubMed
-
- Tannock IF, Rotin D. Cancer Res. 1989;49:4373. - PubMed
-
- Vaupel P, Kallinowski F, Okunieff P. Cancer Res. 1989;49:6449. - PubMed
-
- Wike-Hooley JL, Haveman J, Reinhold JS. Radioter. Oncol. 1984;2:343. - PubMed
-
- Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chem. Rev. 1999;99:2293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

