Natural polyphenol disposition via coupled metabolic pathways
- PMID: 17539746
- PMCID: PMC2777985
- DOI: 10.1517/17425255.3.3.389
Natural polyphenol disposition via coupled metabolic pathways
Abstract
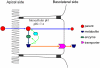
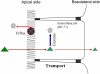
A major challenge associated with the development of chemopreventive polyphenols is the lack of bioavailability in vivo, which are primarily the result of coupled metabolic activities of conjugating enzymes and efflux transporters. These coupling processes are present in disposition tissues and organs in mammals and are efficient for the purposes of drug metabolism, elimination and detoxification. Therefore, it was expected that these coupling processes represent a significant barrier to the oral bioavailabilities of polyphenols. In various studies of this coupling process, it was identified that various conjugating enzymes such as uridine 5'-diphosphate-glucuronosyltransferase and sulfotransferase are capable of producing very hydrophilic metabolites of polyphenols, which cannot diffuse out of the cells and needs the action of efflux transporters to pump them out of the cells. Additional studies have shown that efflux transporters, such as multi-drug resistance-associated protein 2, breast cancer-resistant protein and the organic anion transporters, appear to serve as the gate keeper when there is an excess capacity to metabolise the compounds. These efflux transporters may also act as the facilitator of metabolism when there is a product/metabolite inhibition. For polyphenols, these coupled processes enable a duo recycling scheme of enteric and enterohepatic recycling, which allows the polyphenols to be reabsorbed and results in longer than expected apparent plasma half-lifes for these compounds and their conjugates. Because the vast majority of polyphenols in plasma are hydrophilic conjugates, more research is needed to determine if the metabolites are active or reactive, which will help explain their mechanism of actions.
Figures





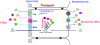
References
-
- MEYER UA. Overview of enzymes of drug metabolism. J. Pharmacokinet. Biopharm. 1996;24(5):449–459. - PubMed
-
- WANG H, LECLUYSE EL. Role of orphan nuclear receptors in the regulation of drug-metabolizing enzymes. Clin. Pharmacokinet. 2003;42(15):1331–13357. - PubMed
-
- BANOGLU E. Current status of the cytosolic sulfotransferases in the metabolic activation of promutagens and procarcinogens. Curr. Drug Metab. 2000;1(1):1–30. - PubMed
-
- INNOCENTI F, GRIMSLEY C, DAS S, et al. Haplotype structure of the UDP-glucuronosyltransferases 1A1 promoter in different ethnic groups. Pharmacogenetics. 2002;12:725–733. - PubMed
-
- MANDLEKAR S, HONG JL, KONG AN. Modulation of metabolic enzymes by dietary phytochemicals: a review of mechanisms underlying beneficial versus unfavorable effects. Curr. Drug Metab. 2006;7(6):661–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
