Cell type-autonomous and non-autonomous requirements for Dmrt1 in postnatal testis differentiation
- PMID: 17540358
- PMCID: PMC1995593
- DOI: 10.1016/j.ydbio.2007.04.046
Cell type-autonomous and non-autonomous requirements for Dmrt1 in postnatal testis differentiation
Abstract
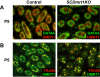
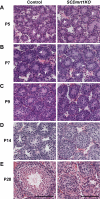
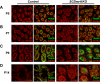
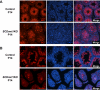
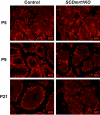
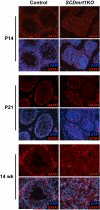
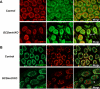
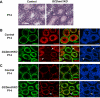
Genes containing the DM domain, a conserved DNA binding motif first found in Doublesex of Drosophila and mab-3 of Caenorhabditis elegans, regulate sexual differentiation in multiple phyla. The DM domain gene Dmrt1 is essential for testicular differentiation in vertebrates. In the mouse, Dmrt1 is expressed in pre-meiotic germ cells and in Sertoli cells, which provide essential support for spermatogenesis. Dmrt1 null mutant mice have severely dysgenic testes in which Sertoli cells and germ cells both fail to differentiate properly after birth. Here we use conditional gene targeting to identify the functions of Dmrt1 in each cell type. We find that Dmrt1 is required in Sertoli cells for their postnatal differentiation, and for germ line maintenance and for meiotic progression. Dmrt1 is required in germ cells for their radial migration to the periphery of the seminiferous tubule where the spermatogenic niche will form, for mitotic reactivation and for survival beyond the first postnatal week. Thus Dmrt1 activity is required autonomously in the Sertoli and germ cell lineages, and Dmrt1 activity in Sertoli cells is also required non-autonomously to maintain the germ line. These results demonstrate that Dmrt1 plays multiple roles in controlling the remodeling and differentiation of the juvenile testis.
Figures

References
-
- Adams IR, McLaren A. Sexually dimorphic development of mouse primordial germ cells: switching from oogenesis to spermatogenesis. Development. 2002;129:1155–64. - PubMed
-
- Anderson R, Copeland TK, Scholer H, Heasman J, Wylie C. The onset of germ cell migration in the mouse embryo. Mech Dev. 2000;91:61–8. - PubMed
-
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. - PubMed
-
- Bremner WJ, Millar MR, Sharpe RM, Saunders PT. Immunohistochemical localization of androgen receptors in the rat testis: evidence for stage-dependent expression and regulation by androgens. Endocrinology. 1994;135:1227–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

