Cyclin box structure of the P-TEFb subunit cyclin T1 derived from a fusion complex with EIAV tat
- PMID: 17540406
- PMCID: PMC1987359
- DOI: 10.1016/j.jmb.2007.04.077
Cyclin box structure of the P-TEFb subunit cyclin T1 derived from a fusion complex with EIAV tat
Abstract
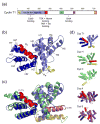
The positive transcription elongation factor b (P-TEFb) is an essential regulator of viral gene expression during the life cycle of human immunodeficiency virus type 1 (HIV-1). Its cyclin T1 subunit forms a ternary complex with the viral transcriptional transactivator (Tat) protein and the transactivation response (TAR) RNA element thereby activating cyclin dependent kinase 9 (Cdk9), which stimulates transcription at the level of chain elongation. We report the structure of the cyclin box domain of human cyclin T1 at a resolution of 2.67 A. The structure was obtained by crystallographic analysis of a fusion protein composed of cyclin T1 linked to the transactivator protein Tat from equine infectious anemia virus (EIAV), which is functionally and structurally related to HIV-1 Tat. The conserved cyclin box domain of cyclin T1 exhibits structural features for interaction with physiological binding partners such as Cdk9. A recognition site for Cdk/Cyclin substrates is partly covered by a cyclin T-specific insert, suggesting specific interactions with regulatory factors. The previously identified Tat/TAR recognition motif (TRM) forms a C-terminal helix that is partly occluded in the cyclin box repeat interface, while cysteine 261 is accessible to form an intermolecular zinc finger with Tat. Residues of the TRM contribute to a positively charged groove that may directly attract RNA molecules. The EIAV Tat protein instead appeared undefined from the electron density map suggesting that it is highly disordered. Functional experiments confirmed the TAR binding properties of the fusion protein and suggested residues on the second cyclin box repeat to contribute to Tat stimulated transcription.
Figures



References
-
- Jones KA, Peterlin BM. Control of RNA initiation and elongation at the HIV-1 promoter. Annu Rev Biochem. 1994;63:717–743. - PubMed
-
- Wei P, Garber ME, Fang SM, Fischer WH, Jones KA. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell. 1998;92:451–462. - PubMed
-
- Murray AW. Recycling the cell cycle: cyclins revisited. Cell. 2004;116:221–234. - PubMed
-
- Shilatifard A, Conaway RC, Conaway JW. The RNA polymerase II elongation complex. Annu Rev Biochem. 2003;72:693–715. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

