Evidence that the heme regulatory motifs in heme oxygenase-2 serve as a thiol/disulfide redox switch regulating heme binding
- PMID: 17540772
- PMCID: PMC3957417
- DOI: 10.1074/jbc.M700664200
Evidence that the heme regulatory motifs in heme oxygenase-2 serve as a thiol/disulfide redox switch regulating heme binding
Abstract
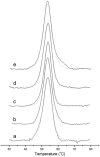
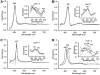
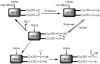
Heme oxygenase (HO) catalyzes the O(2)- and NADPH-dependent conversion of heme to biliverdin, CO, and iron. The two forms of HO (HO-1 and HO-2) share similar physical properties but are differentially regulated and exhibit dissimilar physiological roles and tissue distributions. Unlike HO-1, HO-2 contains heme regulatory motifs (HRMs) (McCoubrey, W. K., Jr., Huang, T. J., and Maines, M. D. (1997) J. Biol. Chem. 272, 12568-12574). Here we describe UV-visible, EPR, and differential scanning calorimetry experiments on human HO-2 variants containing single, double, and triple mutations in the HRMs. Oxidized HO-2, which contains an intramolecular disulfide bond linking Cys(265) of HRM1 and Cys(282) of HRM2, binds heme tightly. Reduction of the disulfide bond increases the K(d) for ferric heme from 0.03 to 0.3 microm, which is much higher than the concentration of the free heme pool in cells. Although the HRMs markedly affect the K(d) for heme, they do not alter the k(cat) for heme degradation and do not bind additional hemes. Because HO-2 plays a key role in CO generation and heme homeostasis, reduction of the disulfide bond would be expected to increase intracellular free heme and decrease CO concentrations. Thus, we propose that the HRMs in HO-2 constitute a thiol/disulfide redox switch that regulates the myriad physiological functions of HO-2, including its involvement in the hypoxic response in the carotid body, which involves interactions with a Ca(2+)-activated potassium channel.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

