Akt-mediated activation of HIF-1 in pulmonary vascular endothelial cells by S-nitrosoglutathione
- PMID: 17541013
- PMCID: PMC1994227
- DOI: 10.1165/rcmb.2006-0289SM
Akt-mediated activation of HIF-1 in pulmonary vascular endothelial cells by S-nitrosoglutathione
Abstract
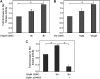
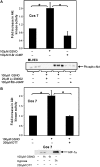
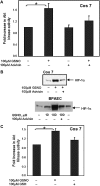
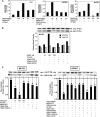
S-nitrosoglutathione (GSNO) stabilizes the alpha-subunit of hypoxia inducible factor-1 (HIF-1) in normoxic cells, but not in the presence of PI3K inhibitors. In this report, the biochemical pathway by which GSNO alters PI3K/Akt activity to modify HIF-1 expression was characterized in Cos cells and primary pulmonary vascular endothelial cells. GSNO increased Akt kinase activity--and downstream HIF-1alpha protein accumulation and DNA-binding activity--in a dose- and time-dependent manner. The PI3K inhibitors, wortmannin and LY294002, blocked these responses. Neither glutathione nor 8-bromo-cyclic GMP mimicked the GSNO-induced increases in Akt kinase activity. GSNO-induced Akt kinase activity and downstream HIF-1alpha stabilization were blocked by acivicin, an inhibitor of gamma-glutamyl transpeptidase (gammaGT), a transmembrane protein that can translate extracellular GSNO to intracellular S-nitrosocysteinylglycine. Dithiothreitol blocked GSNO-induced Akt kinase activity and HIF-1alpha stabilization. Moreover, the 3'-phosphatase of phosphoinositides, PTEN (phosphatase and tensin homolog deleted on chromosome ten) was S-nitrosylated by GSNO in pulmonary arterial endothelial cells, which was reversed by dithiothreitol and ultraviolet light. Interestingly, the abundance of S-nitrosylated PTEN also correlated inversely with PTEN activity. Taken together, these results suggest that GSNO induction of Akt appears to be mediated by S-nitrosylation chemistry rather than classic NO signaling through guanylate cyclase/cGMP. We speculate that gammaGT-dependent activation of Akt and subsequent activation of HIF-1 in vascular beds may be relevant to the regulation of HIF-1-dependent gene expression in conditions associated with oxyhemoglobin deoxygenation, as opposed to profoundly low Po(2), in the pulmonary vasculature.
Figures


References
-
- Semenza GL. O2-regulated gene expression: transcriptional control of cardiorespiratory physiology by HIF-1. J Appl Physiol 2004;96:1173–1177. - PubMed
-
- Zhou J, Callapina M, Goodall GJ, Brune B. Functional integrity of nuclear factor kappa B, phosphatidylinositol 3′-kinase, and mitogen-activated protein kinase signaling allows tumor necrosis factor alpha-evoked Bcl-2 expression to provoke internal ribosome entry site-dependent translation of hypoxia-inducible factor 1alpha. Cancer Res 2004;64:9041–9048. - PubMed
-
- Qian D, Lin HY, Wang HM, Zhang X, Liu DL, Li QL, Zhu C. Normoxic induction of the hypoxic-inducible factor-1 alpha by interleukin-1 beta involves the extracellular signal-regulated kinase 1/2 pathway in normal human cytotrophoblast cells. Biol Reprod 2004;70:1822–1827. - PubMed
-
- Kasuno K, Takabuchi S, Fukuda K, Kizaka-Kondoh S, Yodoi J, Adachi T, Semenza GL, Hirota K. Nitric oxide induces hypoxia-inducible factor 1 activation that is dependent on MAPK and phosphatidylinositol 3-kinase signaling. J Biol Chem 2004;279:2550–2558. - PubMed
-
- Palmer LA, Gaston B, Johns RA. Normoxic stabilization of hypoxia-inducible factor-1 expression and activity: redox-dependent effect of nitrogen oxides. Mol Pharmacol 2000;58:1197–1203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

