Interfering with disease: a progress report on siRNA-based therapeutics
- PMID: 17541417
- PMCID: PMC7098199
- DOI: 10.1038/nrd2310
Interfering with disease: a progress report on siRNA-based therapeutics
Abstract
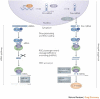
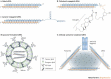
RNA interference (RNAi) quietly crept into biological research in the 1990s when unexpected gene-silencing phenomena in plants and flatworms first perplexed scientists. Following the demonstration of RNAi in mammalian cells in 2001, it was quickly realized that this highly specific mechanism of sequence-specific gene silencing might be harnessed to develop a new class of drugs that interfere with disease-causing or disease-promoting genes. Here we discuss the considerations that go into developing RNAi-based therapeutics starting from in vitro lead design and identification, to in vivo pre-clinical drug delivery and testing. We conclude by reviewing the latest clinical experience with RNAi therapeutics.
Conflict of interest statement
Antonin de Fougerolles, Hans-Peter Vornlocher and John Maraganore are employees of Alnylam Pharmaceuticals and have financial interests in Alnylam. Judy Lieberman is a member of the Scientific Advisory Board of Alnylam.
Figures

References
-
- Elbashir SM, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. - PubMed
-
- Song E, et al. RNA interference targeting Fas protects mice from fulminant hepatitis. Nature Med. 2003;9:347–351. - PubMed
-
- Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–349. - PubMed
-
- Kim DH, Rossi JJ. Strategies for silencing human disease using RNA interference. Nature Rev. Genet. 2007;8:173–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

