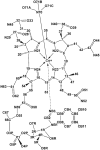
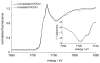
Nitroxylcob(III)alamin: synthesis and X-ray structural characterization
- PMID: 17542034
- PMCID: PMC2764306
- DOI: 10.1002/anie.200701131
Nitroxylcob(III)alamin: synthesis and X-ray structural characterization
Figures

Similar articles
-
Aura of corroles.Chemistry. 2009 Aug 24;15(34):8382-8394. doi: 10.1002/chem.200900920. Chemistry. 2009. PMID: 19630016
-
First syntheses and X-ray structures of a meso-alkyl-substituted corrole and its Ga(III) complex.J Inorg Biochem. 2000 Jul 1;80(3-4):235-8. doi: 10.1016/s0162-0134(00)00077-5. J Inorg Biochem. 2000. PMID: 11001094
-
Characterization of chlorovinylcobalamin, a putative intermediate in reductive degradation of chlorinated ethylenes.J Am Chem Soc. 2003 Apr 16;125(15):4410-1. doi: 10.1021/ja029692c. J Am Chem Soc. 2003. PMID: 12683797
-
Spin canting and metamagnetism in a 3D homometallic molecular material constructed by interpenetration of two kinds of cobalt(II)-coordination-polymer sheets.Angew Chem Int Ed Engl. 2005 May 13;44(20):3079-82. doi: 10.1002/anie.200462463. Angew Chem Int Ed Engl. 2005. PMID: 15770632 No abstract available.
-
Co(II)/Co(I) reduction-induced axial histidine-flipping in myoglobin reconstituted with a cobalt tetradehydrocorrin as a methionine synthase model.Chem Commun (Camb). 2014 Oct 25;50(83):12560-3. doi: 10.1039/c4cc05448b. Chem Commun (Camb). 2014. PMID: 25197974
Cited by
-
Accurate assessment and identification of naturally occurring cellular cobalamins.Clin Chem Lab Med. 2008;46(12):1739-46. doi: 10.1515/CCLM.2008.356. Clin Chem Lab Med. 2008. PMID: 18973458 Free PMC article.
-
Coordination of non-innocent nitrogen oxide ligands: terminology.J Biol Inorg Chem. 2019 May;24(3):315-316. doi: 10.1007/s00775-019-01659-0. Epub 2019 Apr 29. J Biol Inorg Chem. 2019. PMID: 31037467
-
Mechanistic Studies on the Reaction of Nitrocobalamin with Glutathione: Kinetic evidence for formation of an aquacobalamin intermediate.Eur J Inorg Chem. 2013 Jun 1;2013(17):10.1002/ejic.201300254. doi: 10.1002/ejic.201300254. Eur J Inorg Chem. 2013. PMID: 24415907 Free PMC article.
-
Can nitrocobalamin be reduced by ascorbic acid to nitroxylcobalamin? Some surprising mechanistic findings.J Biol Inorg Chem. 2018 May;23(3):377-383. doi: 10.1007/s00775-018-1540-1. Epub 2018 Feb 12. J Biol Inorg Chem. 2018. PMID: 29435646 Free PMC article.
-
NMR spectroscopy and molecular modelling studies of nitrosylcobalamin: further evidence that the deprotonated, base-off form is important for nitrosylcobalamin in solution.Dalton Trans. 2009 Jan 21;(3):424-33. doi: 10.1039/b810895a. Epub 2008 Dec 5. Dalton Trans. 2009. PMID: 19122899 Free PMC article.
References
-
- Bian K, Murad F. Front. Biosci. 2003;8:d264–d278. - PubMed
-
-
The terms “nitric oxide” and “nitric oxide donors” can refer to other nitrogen monoxide species (NO+ and NO−) in addition to ·NO.
-
-
- Nicolaou A, Kenyon SH, Gibbons JM, Ast T, Gibbons WA. Eur. J. Clin. Invest. 1996;26:167–170. - PubMed
-
- Kambo A, Sharma VS, Casteel DE, Woods VL, Jr., Pilz RB, Boss GR. J. Biol. Chem. 2005;280:10073–10082. - PubMed
-
- Brouwer M, Chamulitrat W, Ferruzzi G, Sauls DL, Weinberg JB. Blood. 1996;88:1857–1864. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

