Exploiting ligand conformation in selective inhibition of non-ribosomal peptide synthetase amino acid adenylation with designed macrocyclic small molecules
- PMID: 17542590
- PMCID: PMC2565600
- DOI: 10.1021/ja0721521
Exploiting ligand conformation in selective inhibition of non-ribosomal peptide synthetase amino acid adenylation with designed macrocyclic small molecules
Abstract
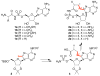
Macrocyclic aminoacyl-AMP analogs have been developed to inhibit non-ribosomal peptide synthetase amino acid adenylation domains selectively by mimicking a cisoid ligand binding conformation observed in crystal structures. In contrast, these macrocycles do not inhibit aminoacyl-tRNA synthetases, which are mechanistically closely related but bind their ligands in a distinct transoid conformation. The macrocycles contain a two- or three-carbon linker between Cβ of the amino acid moiety and C8 of the adenine ring and a sulfamate in place of the phosphate group. These compounds are potent inhibitors of the cysteine adenylation domain activity of the yersiniabactin siderophore synthetase HMWP2 and, unlike the corresponding linear aminoacyl-AMP analogs, do not inhibit protein translation in vitro. Selective small molecule inhibitors of non-ribosomal peptide synthesis should provide a powerful means to study the biological functions of non-ribosomal peptide natural products and a potential avenue to develop novel antibiotics.
Figures

References
-
- Keller L, Surette MG. Nat Rev Microbiol. 2006;4:249–258. - PubMed
-
- Ratledge C, Dover LG. Annu Rev Microbiol. 2000;54:881–941. - PubMed
-
- Visca P, Imperi F, Lamont IL. Trends Microbiol. 2007;15:22–30. - PubMed
- Stein T. Mol Microbiol. 2005;56:845–857. - PubMed
- Roongsawang N, Hase Ki, Haruki M, Imanaka T, Morikawa M, Kanaya S. Chem Biol. 2003;10:869–880. - PubMed
- Hofemeister J, Conrad B, Adler B, Hofemeister B, Feesche J, Kucheryava N, Steinborn G, Franke P, Grammel N, Zwintscher A, Leenders F, Hitzeroth G, Vater J. Mol Genet Genom. 2004;272:363–378. - PubMed
- Raaijmakers JM, de Bruijn I, de Kock MJD. Mol Plant-Microbe Interact. 2006;19:699–710. - PubMed
- Freeman R, Geier H, Weigel KM, Do J, Ford TE, Cangelosi GA. Appl Environ Microbiol. 2006;72:7554–7558. - PMC - PubMed
-
- Nougayrede JP, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, Buchrieser C, Hacker J, Dobrindt U, Oswald E. Science. 2006;313:848–851. - PubMed
-
- Fischbach MA, Walsh CT. Chem Rev. 2006;106:3468–3496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

