Morphine inhibits doxorubicin-induced reactive oxygen species generation and nuclear factor kappaB transcriptional activation in neuroblastoma SH-SY5Y cells
- PMID: 17542780
- PMCID: PMC1948956
- DOI: 10.1042/BJ20070186
Morphine inhibits doxorubicin-induced reactive oxygen species generation and nuclear factor kappaB transcriptional activation in neuroblastoma SH-SY5Y cells
Abstract
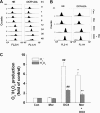
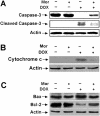
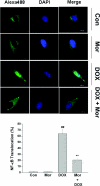
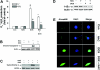
Morphine is recommended as a first-line opioid analgesic in the pain management of cancer patients. Accumulating evidence shows that morphine has anti-apoptotic activity, but its impact on the therapeutic applications of antineoplastic drugs is not well known. The present study was undertaken to test the hypothesis that morphine might antagonize the pro-apoptotic activity of DOX (doxorubicin), a commonly used antitumour drug for the treatment of neuroblastoma, in cultured SH-SY5Y cells. In the present study we demonstrated that morphine suppressed DOX-induced inhibition of cell proliferation and programmed cell death in a concentration-dependent, and naloxone as well as pertussis toxin-irreversible, manner. Further studies showed that morphine inhibited ROS (reactive oxygen species) generation, and prevented DOX-mediated caspase-3 activation, cytochrome c release and changes of Bax and Bcl-2 protein expression. The antioxidant NAC (N-acetylcysteine) also showed the same effects as morphine on DOX-induced ROS generation, caspase-3 activation and cytochrome c release and changes in Bax (Bcl-2-associated X protein) and Bcl-2 protein expression. Additionally, morphine was found to suppress DOX-induced NF-kappaB (nuclear factor kappaB) transcriptional activation via a reduction of IkappaBalpha (inhibitor of nuclear factor kappaB) degradation. These present findings support the hypothesis that morphine can inhibit DOX-induced neuroblastoma cell apoptosis by the inhibition of ROS generation and mitochondrial cytochrome c release, as well as by blockade of NF-kappaB transcriptional activation, and suggests that morphine might have an impact on the antitumour efficiency of DOX.
Figures


References
-
- Ryan M., Moynihan T. J., Loprinzi C. L. As-needed morphine: yes, but at what dose and at what interval? J. Clin. Oncol. 2005;23:3849–3852. - PubMed
-
- Davis M. P., Walsh D., Lagman R., LeGrand S. B. Controversies in pharmacotherapy of pain management. Lancet Oncol. 2005;6:696–704. - PubMed
-
- Meriney S. D., Gray D. B., Pilar G. Morphine-induced delay of normal cell death in the avian ciliary ganglion. Science. 1985;228:1451–1453. - PubMed
-
- Kim M. S., Cheong Y. P., So H. S., Lee K. M., Kim T. Y., Oh J., Chung Y. T., Son Y., Kim B. R., Park R. Protective effects of morphine in peroxynitrite-induced apoptosis of primary rat neonatal astrocytes: potential involvement of G protein and phosphatidylinositol 3-kinase (PI3 kinase) Biochem. Pharmacol. 2001;61:779–786. - PubMed
-
- Ishikawa M., Tanno K., Kamo A., Takayanagi Y., Sasaki K. Enhancement of tumor growth by morphine and its possible mechanism in mice. Biol. Pharm. Bull. 1993;16:762–766. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

