The integrins
- PMID: 17543136
- PMCID: PMC1929136
- DOI: 10.1186/gb-2007-8-5-215
The integrins
Abstract
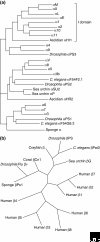
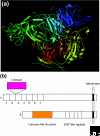
The integrins are a superfamily of cell adhesion receptors that bind to extracellular matrix ligands, cell-surface ligands, and soluble ligands. They are transmembrane alphabeta heterodimers and at least 18 alpha and eight beta subunits are known in humans, generating 24 heterodimers. Members of this family have been found in mammals, chicken and zebrafish, as well as lower eukaryotes, including sponges, the nematode Caenorhabditis elegans (two alpha and one beta subunits, generating two integrins) and the fruitfly Drosophila melanogaster (five alpha and one beta, generating five integrins). The alpha and beta subunits have distinct domain structures, with extracellular domains from each subunit contributing to the ligand-binding site of the heterodimer. The sequence arginine-glycine-aspartic acid (RGD) was identified as a general integrin-binding motif, but individual integrins are also specific for particular protein ligands. Immunologically important integrin ligands are the intercellular adhesion molecules (ICAMs), immunoglobulin superfamily members present on inflamed endothelium and antigen-presenting cells. On ligand binding, integrins transduce signals into the cell interior; they can also receive intracellular signals that regulate their ligand-binding affinity. Here we provide a brief overview that concentrates mostly on the organization, structure and function of mammalian integrins, which have been more extensively studied than integrins in other organisms.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

