Cellular immune response to an engineered cell-based tumor vaccine at the vaccination site
- PMID: 17543914
- PMCID: PMC1949498
- DOI: 10.1016/j.cellimm.2007.04.004
Cellular immune response to an engineered cell-based tumor vaccine at the vaccination site
Abstract
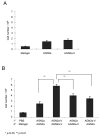
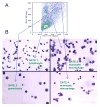
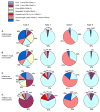
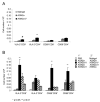
The engineered expression of the immune co-stimulatory molecules CD80 and CD137L on the surface of a neuroblastoma cell line converts this tumor into a cell-based cancer vaccine. The mechanism by which this vaccine activates the immune system was investigated by capturing and analyzing immune cells responding to the vaccine cell line embedded in a collagen matrix and injected subcutaneously. The vaccine induced a significant increase in the number of activated CD62L(-) CCR7(-) CD49b(+) CD8 effector memory T cells captured in the matrix. Importantly, vaccine responsive cells could be detected in the vaccine matrix within a matter of days as demonstrated by IFN-gamma production. The substitution of unmodified tumor cells for the vaccine during serial vaccination resulted in a significant decrease in activated T cells present in the matrix, indicating that immune responses at the vaccine site are a dynamic process that must be propagated by continued co-stimulation.
Figures



Similar articles
-
Induction of a VLA-2 (CD49b)-expressing effector T cell population by a cell-based neuroblastoma vaccine expressing CD137L.J Immunol. 2008 Oct 1;181(7):4621-31. doi: 10.4049/jimmunol.181.7.4621. J Immunol. 2008. PMID: 18802064 Free PMC article.
-
Murine CD8 lymphocyte expansion in vitro by artificial antigen-presenting cells expressing CD137L (4-1BBL) is superior to CD28, and CD137L expressed on neuroblastoma expands CD8 tumour-reactive effector cells in vivo.Immunology. 2004 May;112(1):105-16. doi: 10.1111/j.1365-2567.2004.01853.x. Immunology. 2004. PMID: 15096190 Free PMC article.
-
Development of a potent melanoma vaccine capable of stimulating CD8(+) T-cells independently of dendritic cells in a mouse model.Cancer Immunol Immunother. 2015 Jul;64(7):861-72. doi: 10.1007/s00262-015-1695-3. Epub 2015 Apr 19. Cancer Immunol Immunother. 2015. PMID: 25893808 Free PMC article.
-
Induction of autoimmunity by expansion of autoreactive CD4+CD62Llow cells in vivo.J Immunol. 2006 Oct 1;177(7):4384-90. doi: 10.4049/jimmunol.177.7.4384. J Immunol. 2006. PMID: 16982873
-
4-1BB ligand enhances tumor-specific immunity of poxvirus vaccines.Vaccine. 2006 Jun 5;24(23):4975-86. doi: 10.1016/j.vaccine.2006.03.042. Epub 2006 Mar 31. Vaccine. 2006. PMID: 16621183 Free PMC article.
Cited by
-
Examining T cells at vaccine sites of tumor-bearing hosts provides insights to dysfunctional T-cell immunity.J Immunother. 2013 Jan;36(1):41-51. doi: 10.1097/CJI.0b013e318274590e. J Immunother. 2013. PMID: 23211619 Free PMC article.
-
Skin-Integrated Electrogenetic Regulation of Vasculature for Accelerated Wound Healing.Adv Sci (Weinh). 2025 Mar;12(9):e2412257. doi: 10.1002/advs.202412257. Epub 2025 Jan 10. Adv Sci (Weinh). 2025. PMID: 39792704 Free PMC article.
-
Expression of macrophage migration inhibitory factor by neuroblastoma leads to the inhibition of antitumor T cell reactivity in vivo.J Immunol. 2008 Aug 1;181(3):1877-86. doi: 10.4049/jimmunol.181.3.1877. J Immunol. 2008. PMID: 18641325 Free PMC article.
References
-
- Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT, Brodeur GM, Gerbing RB, Reynolds CP. New Engl J Med. 1999;341:1165–1173. - PubMed
-
- Johnson BD, Gershan JA, Natalia N, Zujewski H, Weber JJ, Yan X, Orentas RJ. J Immunother. 2005;28:449–460. - PubMed
-
- Johnson BD, Yan X, Schauer DW, Orentas RJ. Cell Immunol. 2003;222:15–26. - PubMed
-
- Corthay A, Skovseth DK, Lundin KU, Røsjø E, Omholt H, Hofgaard PO, Haraldsen G, Bogen B. Immunity. 2005;22:371–383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

