Smooth muscle cell signal transduction: implications of vascular biology for vascular surgeons
- PMID: 17544020
- PMCID: PMC1939976
- DOI: 10.1016/j.jvs.2007.02.061
Smooth muscle cell signal transduction: implications of vascular biology for vascular surgeons
Abstract
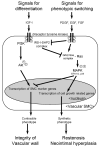
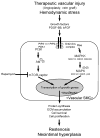
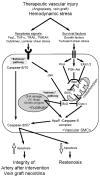
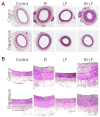
Vascular smooth muscle cells exhibit varied responses after vessel injury and surgical interventions, including phenotypic switching, migration, proliferation, protein synthesis, and apoptosis. Although the source of the smooth muscle cells that accumulate in the vascular wall is controversial, possibly reflecting migration from the adventitia, from the circulating blood, or in situ differentiation, the intracellular signal transduction pathways that control these processes are being defined. Some of these pathways include the Ras-mitogen-activated protein kinase, phosphatidylinositol 3-kinase-Akt, Rho, death receptor-caspase, and nitric oxide pathways. Signal transduction pathways provide amplification, redundancy, and control points within the cell and culminate in biologic responses. We review some of the signaling pathways activated within smooth muscle cells that contribute to smooth muscle cell heterogeneity and development of pathology such as restenosis and neointimal hyperplasia.
Figures
References
-
- Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. - PubMed
-
- Kockx MM, Knaapen MW. The role of apoptosis in vascular disease. J Pathol. 2000;190:267–280. - PubMed
-
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. - PubMed
-
- Schwartz SM, Heimark RL, Majesky MW. Developmental mechanisms underlying pathology of arteries. Physiol Rev. 1990;70:1177–1209. - PubMed
-
- Mitra AK, Gangahar DM, Agrawal DK. Cellular, molecular and immunological mechanisms in the pathophysiology of vein graft intimal hyperplasia. Immunol Cell Biol. 2006;84:115–124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

