Tissue engineering applications to vascular bypass graft development: the use of adipose-derived stem cells
- PMID: 17544030
- PMCID: PMC1941665
- DOI: 10.1016/j.jvs.2007.02.046
Tissue engineering applications to vascular bypass graft development: the use of adipose-derived stem cells
Abstract
The burgeoning field of vascular tissue engineering holds promise for the creation of a practical and successful small-diameter arterial bypass graft. Many creative combinations of autologous cells and scaffolds exist along with an equally long list of microenvironmental cues used to create a functional arterial conduit. This review outlines our work using abdominal wall fat as a source of autologous stem cells for vascular tissue engineering, focusing specifically on this stem cell's availability and potency to differentiate into endothelial-like cells. In a series of 49 patients undergoing elective peripheral vascular surgery, an abundant quantity of adult stem cells was harvested from fat obtained by liposuction. The efficacy of the isolation did not appear influenced by advanced age, obesity, renal failure, or vascular disease, although fat from diabetic patients yielded significantly less stem cells. In addition, these adipose-derived stem cells acquired several morphologic and molecular endothelial phenotypes when exposed to growth factors (endothelial cell growth supplement and vascular endothelial growth factor) and physiologic shear stress in vitro. Taken together, these studies suggest that fat appears to be a viable source of autologous stem cells for use in vascular tissue engineering.
Figures
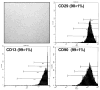

References
-
- Riha GM, Lin PH, Lumsden AB, Yao Q, Chen C. Application of stem cells for vascular tissue engineering. Tissue Engineering. 2005;11:1535–52. - PubMed
-
- Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;87:397–400. - PubMed
-
- Isenberg BC, Williams C, Tranquillo RT. Small-diameter artificial arteries engineered in vitro. Circ Res. 2006;98:25–35. - PubMed
-
- Barrilleaux B, Phinney DG, Prockop Darwin, O’Connor KC. Review: Ex vivo engineering of living tissues with adult stem cells. Tissue Engineering. 2006;12:1–13. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

