Limitations of rapid HIV-1 tests during screening for trials in Uganda: diagnostic test accuracy study
- PMID: 17545184
- PMCID: PMC1934458
- DOI: 10.1136/bmj.39210.582801.BE
Limitations of rapid HIV-1 tests during screening for trials in Uganda: diagnostic test accuracy study
Abstract
Objective: To evaluate the limitations of rapid tests for HIV-1.
Design: Diagnostic test accuracy study.
Setting: Rural Rakai, Uganda.
Participants: 1517 males aged 15-49 screened for trials of circumcision for HIV prevention.
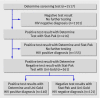
Main outcome measures: Sensitivity, specificity, negative predictive values, and positive predictive values of an algorithm using three rapid tests for HIV, compared with the results of enzyme immunoassay and western blotting as the optimal methods.
Results: Rapid test results were evaluated by enzyme immunoassay and western blotting. Sensitivity was 97.7%. Among 639 samples where the strength of positive bands was coded if the sample showed positivity for HIV, the algorithm had low specificity (94.1%) and a low positive predictive value (74.0%). Exclusion of 37 samples (5.8%) with a weak positive band improved the specificity (99.6%) and positive predictive value (97.7%).
Conclusion: Weak positive bands on rapid tests for HIV should be confirmed by enzyme immunoassay and western blotting before disclosing the diagnosis. Programmes using rapid tests routinely should use standard serological assays for quality control. Trial registration Clinical Trials NCT00425984.
Conflict of interest statement
Figures
Comment in
-
Technological challenges in diagnosis and management of HIV infection in resource limited settings.BMJ. 2007 Jul 28;335(7612):165-6. doi: 10.1136/bmj.39275.457188.AE. BMJ. 2007. PMID: 17656506 Free PMC article.
References
-
- Branson BM. Point-of-care rapid tests for HIV antibody. .www.cdc.gov/hiv/rapid_testing/materials/J_Lab_Med_20031.htm
-
- McKenna SL, Muyinda GK, Roth D, Mwali M, Ng'andu N, Myrick A, et al. Rapid HIV testing and counseling for voluntary testing centers in Africa. AIDS 1997;(Suppl 1):S103-10. - PubMed
-
- Wright AA, Katz IT. Home testing for HIV. N Engl J Med 2006;354:437-40. - PubMed
-
- De Cock KM, Bunnell R, Mermin J. Unfinished business—expanding HIV testing in developing countries: N Engl J Med 2006;354:440-2. - PubMed
-
- Kassler WJ, Alwano-Edyengo MG, Marum E, Biryahwaho B, Kataaha P, Dillon B. Rapid HIV resting with same day results: a field trial in Uganda. Int J STD AIDS 1998;9:134. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical