Structural characterization of ferric hemoglobins from three antarctic fish species of the suborder notothenioidei
- PMID: 17545238
- PMCID: PMC1989692
- DOI: 10.1529/biophysj.107.105700
Structural characterization of ferric hemoglobins from three antarctic fish species of the suborder notothenioidei
Abstract
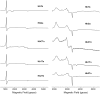
Spontaneous autoxidation of tetrameric Hbs leads to the formation of Fe (III) forms, whose physiological role is not fully understood. Here we report structural characterization by EPR of the oxidized states of tetrameric Hbs isolated from the Antarctic fish species Trematomus bernacchii, Trematomus newnesi, and Gymnodraco acuticeps, as well as the x-ray crystal structure of oxidized Trematomus bernacchii Hb, redetermined at high resolution. The oxidation of these Hbs leads to formation of states that were not usually detected in previous analyses of tetrameric Hbs. In addition to the commonly found aquo-met and hydroxy-met species, EPR analyses show that two distinct hemichromes coexist at physiological pH, referred to as hemichromes I and II, respectively. Together with the high-resolution crystal structure (1.5 A) of T. bernacchii and a survey of data available for other heme proteins, hemichrome I was assigned by x-ray crystallography and by EPR as a bis-His complex with a distorted geometry, whereas hemichrome II is a less constrained (cytochrome b5-like) bis-His complex. In four of the five Antartic fish Hbs examined, hemichrome I is the major form. EPR shows that for HbCTn, the amount of hemichrome I is substantially reduced. In addition, the concomitant presence of a penta-coordinated high-spin Fe (III) species, to our knowledge never reported before for a wild-type tetrameric Hb, was detected. A molecular modeling investigation demonstrates that the presence of the bulkier Ile in position 67beta in HbCTn in place of Val as in the other four Hbs impairs the formation of hemichrome I, thus favoring the formation of the ferric penta-coordinated species. Altogether the data show that ferric states commonly associated with monomeric and dimeric Hbs are also found in tetrameric Hbs.
Figures
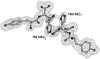
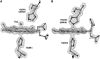

Similar articles
-
Correlation between hemichrome stability and the root effect in tetrameric hemoglobins.Biophys J. 2009 Aug 5;97(3):866-74. doi: 10.1016/j.bpj.2009.04.056. Biophys J. 2009. PMID: 19651045 Free PMC article.
-
Occurrence and formation of endogenous histidine hexa-coordination in cold-adapted hemoglobins.IUBMB Life. 2011 May;63(5):295-303. doi: 10.1002/iub.446. Epub 2011 Apr 13. IUBMB Life. 2011. PMID: 21491555 Review.
-
The oxidation process of Antarctic fish hemoglobins.Eur J Biochem. 2004 May;271(9):1651-9. doi: 10.1111/j.1432-1033.2004.04054.x. Eur J Biochem. 2004. PMID: 15096204
-
Spectroscopic and crystallographic characterization of bis-histidyl adducts in tetrameric hemoglobins.Methods Enzymol. 2008;436:425-44. doi: 10.1016/S0076-6879(08)36024-8. Methods Enzymol. 2008. PMID: 18237647 Review.
-
The crystal structure of a tetrameric hemoglobin in a partial hemichrome state.Proc Natl Acad Sci U S A. 2002 Jul 23;99(15):9801-6. doi: 10.1073/pnas.132182099. Epub 2002 Jul 1. Proc Natl Acad Sci U S A. 2002. PMID: 12093902 Free PMC article.
Cited by
-
Correlation between hemichrome stability and the root effect in tetrameric hemoglobins.Biophys J. 2009 Aug 5;97(3):866-74. doi: 10.1016/j.bpj.2009.04.056. Biophys J. 2009. PMID: 19651045 Free PMC article.
-
Quaternary Structure Transitions of Human Hemoglobin: An Atomic-Level View of the Functional Intermediate States.J Chem Inf Model. 2021 Aug 23;61(8):3988-3999. doi: 10.1021/acs.jcim.1c00315. Epub 2021 Aug 10. J Chem Inf Model. 2021. PMID: 34375114 Free PMC article.
-
Molecular characterization and different expression patterns of the FABP gene family during goat skeletal muscle development.Mol Biol Rep. 2015 Jan;42(1):201-7. doi: 10.1007/s11033-014-3759-4. Epub 2014 Sep 23. Mol Biol Rep. 2015. PMID: 25245957
-
Atomic-Level View of the Functional Transition in Vertebrate Hemoglobins: The Case of Antarctic Fish Hbs.J Chem Inf Model. 2022 Aug 22;62(16):3874-3884. doi: 10.1021/acs.jcim.2c00727. Epub 2022 Aug 5. J Chem Inf Model. 2022. PMID: 35930673 Free PMC article.
-
Crystal structural investigations of heme protein derivatives resulting from reactions of aryl- and alkylhydroxylamines with human hemoglobin.J Inorg Biochem. 2023 Sep;246:112304. doi: 10.1016/j.jinorgbio.2023.112304. Epub 2023 Jun 25. J Inorg Biochem. 2023. PMID: 37406385 Free PMC article.
References
-
- Shikama, K. 1998. The molecular mechanism of autoxidation for myoglobin and hemoglobin: a venerable puzzle. Chem. Rev. 98:1357–1374. - PubMed
-
- Riess, J. 2001. Oxygen carriers (“blood substitutes”) raison d'être, chemistry, and some physiology. Chem. Rev. 101:2797–2919. - PubMed
-
- Rifkind, J. M., O. Abugo, A. Levy, and J. M. Heim. 1994. Detection, formation, and relevance of hemichrome and hemochrome. Meth. Enzymol. 231:449–480. - PubMed
-
- Vitagliano, L., G. Bonomi, A. Riccio, G. di Prisco, G. Smulevich, and L. Mazzarella. 2004. The oxidation process of Antarctic fish hemoglobins. Eur. J. Biochem. 271:1651–1659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

