The first agmatine/cadaverine aminopropyl transferase: biochemical and structural characterization of an enzyme involved in polyamine biosynthesis in the hyperthermophilic archaeon Pyrococcus furiosus
- PMID: 17545282
- PMCID: PMC1952034
- DOI: 10.1128/JB.00151-07
The first agmatine/cadaverine aminopropyl transferase: biochemical and structural characterization of an enzyme involved in polyamine biosynthesis in the hyperthermophilic archaeon Pyrococcus furiosus
Abstract
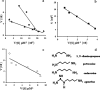
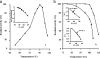
We report here the characterization of the first agmatine/cadaverine aminopropyl transferase (ACAPT), the enzyme responsible for polyamine biosynthesis from an archaeon. The gene PF0127 encoding ACAPT in the hyperthermophile Pyrococcus furiosus was cloned and expressed in Escherichia coli, and the recombinant protein was purified to homogeneity. P. furiosus ACAPT is a homodimer of 65 kDa. The broad substrate specificity of the enzyme toward the amine acceptors is unique, as agmatine, 1,3-diaminopropane, putrescine, cadaverine, and sym-nor-spermidine all serve as substrates. While maximal catalytic activity was observed with cadaverine, agmatine was the preferred substrate on the basis of the k(cat)/K(m) value. P. furiosus ACAPT is thermoactive and thermostable with an apparent melting temperature of 108 degrees C that increases to 112 degrees C in the presence of cadaverine. Limited proteolysis indicated that the only proteolytic cleavage site is localized in the C-terminal region and that the C-terminal peptide is not necessary for the integrity of the active site. The crystal structure of the enzyme determined to 1.8-A resolution confirmed its dimeric nature and provided insight into the proteolytic analyses as well as into mechanisms of thermal stability. Analysis of the polyamine content of P. furiosus showed that spermidine, cadaverine, and sym-nor-spermidine are the major components, with small amounts of sym-nor-spermine and N-(3-aminopropyl)cadaverine (APC). This is the first report in Archaea of an unusual polyamine APC that is proposed to play a role in stress adaptation.
Figures


References
-
- Arendall, W. B., III, W. Tempel, J. S. Richardson, W. Zhou, S. Wang, I. W. Davis, Z. J. Liu, J. P. Rose, W. M. Carson, M. Luo, D. C. Richardson, and B. C. Wang. 2005. A test of enhancing model accuracy in high-throughput crystallography. J. Struct. Funct. Genomics 6:1-11. - PubMed
-
- Bowman, W. H., C. W. Tabor, and H. Tabor. 1973. Spermidine biosynthesis. Purification and properties of propylamine transferase from Escherichia coli. J. Biol. Chem. 248:2480-2486. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

