The intracellular concentration of acetyl phosphate in Escherichia coli is sufficient for direct phosphorylation of two-component response regulators
- PMID: 17545286
- PMCID: PMC1951799
- DOI: 10.1128/JB.00564-07
The intracellular concentration of acetyl phosphate in Escherichia coli is sufficient for direct phosphorylation of two-component response regulators
Abstract
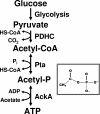
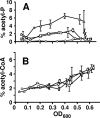
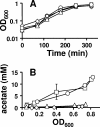
Acetyl phosphate, the intermediate of the AckA-Pta pathway, acts as a global signal in Escherichia coli. Although acetyl phosphate clearly signals through two-component response regulators, it remains unclear whether acetyl phosphate acts as a direct phospho donor or functions through an indirect mechanism. We used two-dimensional thin-layer chromatography to measure the relative concentrations of acetyl phosphate, acetyl coenzyme A, ATP, and GTP over the course of the entire growth curve. We estimated that the intracellular concentration of acetyl phosphate in wild-type cells reaches at least 3 mM, a concentration sufficient to activate two-component response regulators via direct phosphoryl transfer.
Figures


References
-
- Bang, I. S., J. P. Audia, Y. K. Park, and J. W. Foster. 2002. Autoinduction of the ompR response regulator by acid shock and control of the Salmonella enterica acid tolerance response. Mol. Microbiol. 44:1235-1250. - PubMed
-
- Bertagnolli, B. L., and L. P. Hager. 1991. Activation of Escherichia coli pyruvate oxidase enhances the oxidation of hydroxyethylthiamin pyrophosphate. J. Biol. Chem. 266:10168-10173. - PubMed
-
- Bertagnolli, B. L., and L. P. Hager. 1993. Role of flavin in acetoin production by two bacterial pyruvate oxidases. Arch. Biochem. Biophys. 300:364-371. - PubMed
-
- Bochner, B. R., and B. N. Ames. 1982. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J. Biol. Chem. 257:9759-9769. - PubMed
-
- Bochner, B. R., and B. N. Ames. 1982. Selective precipitation orthophosphate from mixtures containing labile phosphorylated metabolites. Anal. Biochem. 122:100-107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

