Cysteine scanning mutagenesis and topological mapping of the Escherichia coli twin-arginine translocase TatC Component
- PMID: 17545291
- PMCID: PMC1951830
- DOI: 10.1128/JB.00647-07
Cysteine scanning mutagenesis and topological mapping of the Escherichia coli twin-arginine translocase TatC Component
Abstract
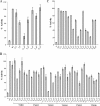
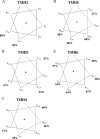
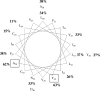
The TatC protein is an essential component of the Escherichia coli twin-arginine (Tat) protein translocation pathway. It is a polytopic membrane protein that forms a complex with TatB, together acting as the receptor for Tat substrates. In this study we have constructed 57 individual cysteine substitutions throughout the protein. Each of the substitutions resulted in a TatC protein that was competent to support Tat-dependent protein translocation. Accessibility studies with membrane-permeant and -impermeant thiol-reactive reagents demonstrated that TatC has six transmembrane helices, rather than the four suggested by a previous study (K. Gouffi, C.-L. Santini, and L.-F. Wu, FEBS Lett. 525:65-70, 2002). Disulfide cross-linking experiments with TatC proteins containing single cysteine residues showed that each transmembrane domain of TatC was able to interact with the same domain from a neighboring TatC protein. Surprisingly, only three of these cysteine variants retained the ability to cross-link at low temperatures. These results are consistent with the likelihood that most of the disulfide cross-links are between TatC proteins in separate TatBC complexes, suggesting that TatC is located on the periphery of the complex.
Figures

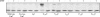

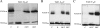


References
-
- Alami, M., I. Luke, S. Deitermann, G. Eisner, H. G. Koch, J. Brunner, and M. Müller. 2003. Differential interactions between a twin-arginine signal peptide and its translocase in Escherichia coli. Mol. Cell 12:937-946. - PubMed
-
- Allen, S. C., C. M. Barrett, N. Ray, and C. Robinson. 2002. Essential cytoplasmic domains in the Escherichia coli TatC protein. J. Biol. Chem. 277:10362-10366. - PubMed
-
- Behrendt, J., K. Standar, U. Lindenstrauss, and T. Brüser. 2004. Topological studies on the twin-arginine translocase component TatC. FEMS Microbiol. Lett. 234:303-308. - PubMed
-
- Berks, B. C., T. Palmer, and F. Sargent. 2003. The Tat protein translocation pathway and its role in microbial physiology. Adv. Microb. Physiol. 47:187-254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

