Trichoderma atroviride PHR1, a fungal photolyase responsible for DNA repair, autoregulates its own photoinduction
- PMID: 17545314
- PMCID: PMC2043357
- DOI: 10.1128/EC.00208-06
Trichoderma atroviride PHR1, a fungal photolyase responsible for DNA repair, autoregulates its own photoinduction
Abstract
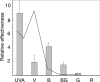
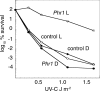
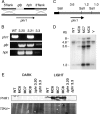
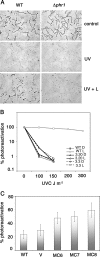
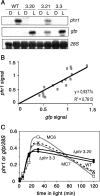
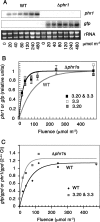
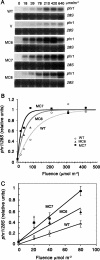
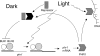
The photolyases, DNA repair enzymes that use visible and long-wavelength UV light to repair cyclobutane pyrimidine dimers (CPDs) created by short-wavelength UV, belong to the larger photolyase-cryptochrome gene family. Cryptochromes (UVA-blue light photoreceptors) lack repair activity, and sensory and regulatory roles have been defined for them in plants and animals. Evolutionary considerations indicate that cryptochromes diverged from CPD photolyases before the emergence of eukaryotes. In prokaryotes and lower eukaryotes, some photolyases might have photosensory functions. phr1 codes for a class I CPD photolyase in Trichoderma atroviride. phr1 is rapidly induced by blue and UVA light, and its photoinduction requires functional blue light regulator (BLR) proteins, which are White Collar homologs in Trichoderma. Here we show that deletion of phr1 abolished photoreactivation of UVC (200 to 280 nm)-inhibited spores and thus that PHR1 is the main component of the photorepair system. The 2-kb 5' upstream region of phr1, with putative light-regulated elements, confers blue light regulation on a reporter gene. To assess phr1 photosensory function, fluence response curves of this light-regulated promoter were tested in null mutant (Deltaphr1) strains. Photoinduction of the phr1 promoter in Deltaphr1 strains was >5-fold more sensitive to light than that in the wild type, whereas in PHR1-overexpressing lines the sensitivity to light increased about 2-fold. Our data suggest that PHR1 may regulate its expression in a light-dependent manner, perhaps through negative modulation of the BLR proteins. This is the first evidence for a regulatory role of photolyase, a role usually attributed to cryptochromes.
Figures


References
-
- Ahmad, M., and A. R. Cashmore. 1993. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366:162-166. - PubMed
-
- Alejandre-Duran, E., T. Roldan-Arjona, R. R. Ariza, and M. RuizRubio. 2003. The photolyase gene from the plant pathogen Fusarium oxyosporum f. sp. lycopersici is induced by visible light and α-tomatine from tomato plant. Fungal Genet. Biol. 40:159-165. - PubMed
-
- Berrocal-Tito, G., T. Rosales-Saavedra, A. Herrera-Estrella, and B. Horwitz. 2000. Characterization of blue-light and developmental regulation of the photolyase gene phr1 in Trichoderma harzianum. Photochem. Photobiol. 71:662-668. - PubMed
-
- Berrocal-Tito, G., L. Sametz-Baron, K. Eichenberg, B. A. Horwitz, and A. Herrera-Estrella. 1999. Rapid light regulation of a Trichoderma harzianum DNA photolyase gene. J. Biol. Chem. 274:14288-14294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

