Quantitative analysis of population heterogeneity of the adaptive salt stress response and growth capacity of Bacillus cereus ATCC 14579
- PMID: 17545319
- PMCID: PMC1951020
- DOI: 10.1128/AEM.00404-07
Quantitative analysis of population heterogeneity of the adaptive salt stress response and growth capacity of Bacillus cereus ATCC 14579
Abstract
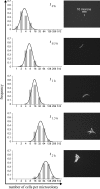
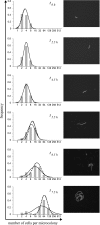
Bacterial populations can display heterogeneity with respect to both the adaptive stress response and growth capacity of individual cells. The growth dynamics of Bacillus cereus ATCC 14579 during mild and severe salt stress exposure were investigated for the population as a whole in liquid culture. To quantitatively assess the population heterogeneity of the stress response and growth capacity at a single-cell level, a direct imaging method was applied to monitor cells from the initial inoculum to the microcolony stage. Highly porous Anopore strips were used as a support for the culturing and imaging of microcolonies at different time points. The growth kinetics of cells grown in liquid culture were comparable to those of microcolonies grown upon Anopore strips, even in the presence of mild and severe salt stress. Exposure to mild salt stress resulted in growth that was characterized by a remarkably low variability of microcolony sizes, and the distributions of the log(10)-transformed microcolony areas could be fitted by the normal distribution. Under severe salt stress conditions, the microcolony sizes were highly heterogeneous, and this was apparently caused by the presence of both a nongrowing and growing population. After discriminating these two subpopulations, it was shown that the variability of microcolony sizes of the growing population was comparable to that of non-salt-stressed and mildly salt-stressed populations. Quantification of population heterogeneity during stress exposure may contribute to an optimized application of preservation factors for controlling growth of spoilage and pathogenic bacteria to ensure the quality and safety of minimally processed foods.
Figures
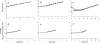


References
-
- Andersson, A., U. Rönner, and P. E. Granum. 1995. What problems does the food industry have with the spore-forming pathogens Bacillus cereus and Clostridium perfringens? Int. J. Food Microbiol. 28:145-155. - PubMed
-
- Anonymous. 2000. @Risk, advanced risk analysis for spreadsheets. Palisade Corp., Newfield, NY.
-
- Avery, S. V. 2006. Microbial cell individuality and the underlying sources of heterogeneity. Nat. Rev. Microbiol. 4:577-587. - PubMed
-
- Booth, I. R. 2002. Stress and the single cell: intrapopulation diversity is a mechanism to ensure survival upon exposure to stress. Int. J. Food Microbiol. 78:19-30. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

