Monitoring and source tracking of tetracycline resistance genes in lagoons and groundwater adjacent to swine production facilities over a 3-year period
- PMID: 17545324
- PMCID: PMC1951052
- DOI: 10.1128/AEM.00665-07
Monitoring and source tracking of tetracycline resistance genes in lagoons and groundwater adjacent to swine production facilities over a 3-year period
Abstract
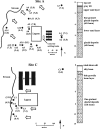
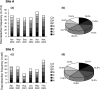
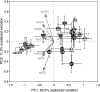
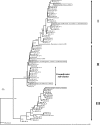
To monitor the dissemination of resistance genes into the environment, we determined the occurrence of tetracycline resistance (Tc(r)) genes in groundwater underlying two swine confinement operations. Monitoring well networks (16 wells at site A and 6 wells at site C) were established around the lagoons at each facility. Groundwater (n = 124) and lagoon (n = 12) samples were collected from the two sites at six sampling times from 2000 through 2003. Total DNA was extracted, and PCR was used to detect seven Tc(r) genes [tet(M), tet(O), tet(Q), tet(W), tet(C), tet(H), and tet(Z)]. The concentration of Tc(r) genes was quantified by real-time quantitative PCR. To confirm the Tc(r) gene source in groundwater, comparative analysis of tet(W) gene sequences was performed on groundwater and lagoon samples. All seven Tc(r) genes were continually detected in groundwater during the 3-year monitoring period at both sites. At site A, elevated detection frequency and concentration of Tc(r) genes were observed in the wells located down-gradient of the lagoon. Comparative analysis of tet(W) sequences revealed that the impacted groundwater contained gene sequences almost identical (99.8% identity) to those in the lagoon, but these genes were not found in background libraries. Novel sequence clusters and unique indigenous resistance gene pools were also found in the groundwater. Thus, antibiotic resistance genes in groundwater are affected by swine manure, but they are also part of the indigenous gene pool.
Figures
References
-
- Aerts, J. L., M. I. Gonzales, and S. L. Topalian. 2004. Selection of appropriate control genes to assess expression of tumor antigens using real-time RT-PCR. BioTechniques 36:84-86. - PubMed
-
- Alonso, A., P. Sánchez, and J. L. Martínez. 2001. Environmental selection of antibiotic resistance genes. Environ. Microbiol. 3:1-9. - PubMed
-
- Amabile-Cuevas, C. F., and M. E. Chicurel. 1992. Bacterial plasmids and gene flux. Cell 70:189-199. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

