Linear distance from the locus control region determines epsilon-globin transcriptional activity
- PMID: 17548470
- PMCID: PMC1952132
- DOI: 10.1128/MCB.00602-07
Linear distance from the locus control region determines epsilon-globin transcriptional activity
Abstract
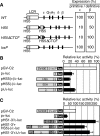
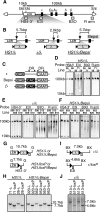
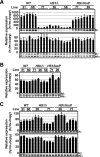
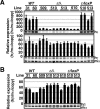
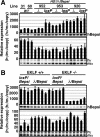
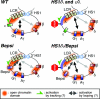
Enhancer elements modulate promoter activity over vast chromosomal distances, and mechanisms that ensure restrictive interactions between promoters and enhancers are critical for proper control of gene expression. The human beta-globin locus control region (LCR) activates expression of five genes in erythroid cells, including the proximal embryonic epsilon- and the distal adult beta-globin genes. To test for possible distance sensitivity of the genes to the LCR, we extended the distance between the LCR and genes by 2.3 kbp within the context of a yeast artificial chromosome, followed by the generation of transgenic mice (TgM). In these TgM lines, epsilon-globin gene expression decreased by 90%, while the more distantly located gamma- or beta-globin genes were not affected. Remarkably, introduction of a consensus EKLF binding site into the epsilon-globin promoter rendered its expression distance insensitive; when tested in an EKLF-null genetic background, expression of the mutant epsilon-globin gene was severely compromised. Thus, the epsilon-globin gene differs in its distance sensitivity to the LCR from the other beta-like globin genes, which is, at least in part, determined by the transcription factor EKLF.
Figures

Similar articles
-
All of the human beta-type globin genes compete for LCR enhancer activity in embryonic erythroid cells of yeast artificial chromosome transgenic mice.FASEB J. 2009 Dec;23(12):4335-43. doi: 10.1096/fj.09-137778. Epub 2009 Aug 18. FASEB J. 2009. PMID: 19690216 Free PMC article.
-
DNase I hypersensitivity and epsilon-globin transcriptional enhancement are separable in locus control region (LCR) HS1 mutant human beta-globin YAC transgenic mice.J Biol Chem. 2010 May 7;285(19):14495-503. doi: 10.1074/jbc.M110.116525. Epub 2010 Mar 15. J Biol Chem. 2010. PMID: 20231293 Free PMC article.
-
Erythroid Krüppel-like factor (EKLF) is active in primitive and definitive erythroid cells and is required for the function of 5'HS3 of the beta-globin locus control region.EMBO J. 1998 Apr 15;17(8):2334-41. doi: 10.1093/emboj/17.8.2334. EMBO J. 1998. PMID: 9545245 Free PMC article.
-
beta-YAC transgenic mice for studying LCR function.Ann N Y Acad Sci. 1998 Jun 30;850:28-37. doi: 10.1111/j.1749-6632.1998.tb10459.x. Ann N Y Acad Sci. 1998. PMID: 9668524 Review.
-
Beyond the locus control region: new light on beta-globin locus regulation.Int J Biochem Cell Biol. 2001 Sep;33(9):914-23. doi: 10.1016/s1357-2725(01)00057-7. Int J Biochem Cell Biol. 2001. PMID: 11461833 Review.
Cited by
-
The role of transcriptional activator GATA-1 at human beta-globin HS2.Nucleic Acids Res. 2008 Aug;36(14):4521-8. doi: 10.1093/nar/gkn368. Epub 2008 Jun 27. Nucleic Acids Res. 2008. PMID: 18586828 Free PMC article.
-
An unusual case of thalassemia intermedia with inheritable complex repeats detected by single-molecule optical mapping.Haematologica. 2024 Mar 1;109(3):1000-1006. doi: 10.3324/haematol.2023.282902. Haematologica. 2024. PMID: 37767576 Free PMC article. Clinical Trial. No abstract available.
-
The H19 imprinting control region mediates preimplantation imprinted methylation of nearby sequences in yeast artificial chromosome transgenic mice.Mol Cell Biol. 2013 Feb;33(4):858-71. doi: 10.1128/MCB.01003-12. Epub 2012 Dec 10. Mol Cell Biol. 2013. PMID: 23230275 Free PMC article.
-
All of the human beta-type globin genes compete for LCR enhancer activity in embryonic erythroid cells of yeast artificial chromosome transgenic mice.FASEB J. 2009 Dec;23(12):4335-43. doi: 10.1096/fj.09-137778. Epub 2009 Aug 18. FASEB J. 2009. PMID: 19690216 Free PMC article.
-
EKLF/KLF1, a tissue-restricted integrator of transcriptional control, chromatin remodeling, and lineage determination.Mol Cell Biol. 2013 Jan;33(1):4-13. doi: 10.1128/MCB.01058-12. Epub 2012 Oct 22. Mol Cell Biol. 2013. PMID: 23090966 Free PMC article. Review.
References
-
- Bell, A. C., A. G. West, and G. Felsenfeld. 1999. The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell 98:387-396. - PubMed
-
- Bulger, M., T. Sawado, D. Schubeler, and M. Groudine. 2002. ChIPs of the beta-globin locus: unraveling gene regulation within an active domain. Curr. Opin. Genet. Dev. 12:170-177. - PubMed
-
- Carter, D., L. Chakalova, C. S. Osborne, Y. F. Dai, and P. Fraser. 2002. Long-range chromatin regulatory interactions in vivo. Nat. Genet. 32:623-626. - PubMed
-
- Choi, O. R., and J. D. Engel. 1988. Developmental regulation of beta-globin gene switching. Cell 55:17-26. - PubMed
-
- Dean, A. 2006. On a chromosome far, far away: LCRs and gene expression. Trends Genet. 22:38-45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
