The secreted pyomelanin pigment of Legionella pneumophila confers ferric reductase activity
- PMID: 17548481
- PMCID: PMC1951983
- DOI: 10.1128/IAI.00489-07
The secreted pyomelanin pigment of Legionella pneumophila confers ferric reductase activity
Abstract
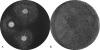
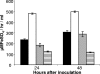
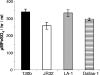
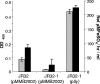
The virulence of Legionella pneumophila is dependent upon its capacity to acquire iron. To identify genes involved in expression of its siderophore, we screened a mutagenized population of L. pneumophila for strains that were no longer able to rescue the growth of a ferrous transport mutant. However, an unusual mutant was obtained that displayed a strong inhibitory effect on the feoB mutant. Due to an insertion in hmgA that encodes homogentisate 1,2-dioxygenase, the mutant secreted increased levels of pyomelanin, the L. pneumophila pigment that is derived from secreted homogentisic acid (HGA). Thus, we hypothesized that L. pneumophila-secreted HGA-melanin has intrinsic ferric reductase activity, converting Fe(3+) to Fe(2+), but that hyperpigmentation results in excessive reduction of iron that can, in the case of the feoB mutant, be inhibitory to growth. In support of this hypothesis, we demonstrated, for the first time, that wild-type L. pneumophila secretes ferric reductase activity. Moreover, whereas the hyperpigmented mutant had increased secreted activity, an lly mutant specifically impaired for pigment production lacked the activity. Compatible with the nature of HGA-melanins, the secreted ferric reductase activity was positively influenced by the amount of tyrosine in the growth medium, resistant to protease, acid precipitable, and heterogeneous in size. Together, these data represent the first demonstration of pyomelanin-mediated ferric reduction by a pathogenic bacterium.
Figures



References
-
- Agodi, A., S. Stefani, C. Corsaro, F. Campanile, S. Gribaldo, and G. Sichel. 1996. Study of a melanic pigment of Proteus mirabilis. Res. Microbiol. 147:167-174. - PubMed
-
- Arias-Barrau, E., E. R. Olivera, J. M. Luengo, C. Fernandez, B. Galan, J. L. Garcia, E. Diaz, and B. Minambres. 2004. The homogentisate pathway: a central catabolic pathway involved in the degradation of l-phenylalanine, l-tyrosine, and 3-hydroxyphenylacetate in Pseudomonas putida. J. Bacteriol. 186:5062-5077. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

