Investigating Toll-like receptor agonists for potential to treat hepatitis C virus infection
- PMID: 17548497
- PMCID: PMC1932540
- DOI: 10.1128/AAC.00268-07
Investigating Toll-like receptor agonists for potential to treat hepatitis C virus infection
Abstract
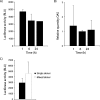
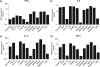
Toll-like receptors (TLRs) are key mediators of innate immunity, and their activation by microbial components leads to the production of cytokines and interferons. Recombinant alpha interferon has been used to treat several viral diseases and is the current standard of care for hepatitis C virus (HCV) infection. Recently, agonists of TLR7 and TLR9 have been shown to have clinical efficacy in HCV patients, and this is correlated with their ability to induce endogenous type I interferon production. We have carried out a comprehensive study of agonists of TLRs 1 to 9 to determine if any additional TLRs can induce antiviral molecules from human peripheral blood mononuclear cells (PBMCs). The agonists were incubated with PBMCs, and the supernatant was then removed and added to HCV replicon cells to assess antiviral activity. Agonists of TLRs 3, 4, 7, 8, and 9 were found to be potent inducers of antiviral activity in PBMC supernatants, and the activity correlated with the induction of alpha interferon and the interferon-induced antiviral biomarker 2',5'-oligoadenylate synthase. Antiviral activity of TLR7 and TLR8 agonists was blocked by an antibody that binds to the type I interferon receptor, confirming that the antiviral activity results from type I interferon induction. TLR4 and TLR8 agonists were found to strongly induce the proinflammatory cytokines interleukin 1beta and tumor necrosis factor alpha at concentrations similar to those inducing antiviral activity. This raises concerns about adverse side effects if these were to be used as antiviral agents. We therefore conclude that TLRs 3, 7, and 9 represent the most attractive targets for the development of new HCV therapies.
Figures






References
-
- Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. - PubMed
-
- Antonelli, G., G. Giannelli, M. Currenti, E. Simeoni, S. Del Vecchio, F. Maggi, M. Pistello, L. Roffi, G. Pastore, L. Chemello, and F. Dianzani. 1996. Antibodies to interferon (IFN) in hepatitis C patients relapsing while continuing recombinant IFN-alpha2 therapy. Clin. Exp. Immunol. 104:384-387. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

