The Ig cell adhesion molecule Basigin controls compartmentalization and vesicle release at Drosophila melanogaster synapses
- PMID: 17548512
- PMCID: PMC2064284
- DOI: 10.1083/jcb.200701111
The Ig cell adhesion molecule Basigin controls compartmentalization and vesicle release at Drosophila melanogaster synapses
Abstract
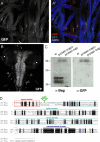
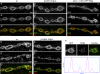
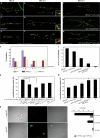
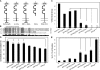
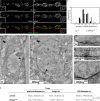
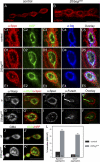
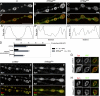
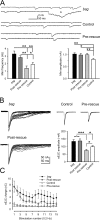
Synapses can undergo rapid changes in size as well as in their vesicle release function during both plasticity processes and development. This fundamental property of neuronal cells requires the coordinated rearrangement of synaptic membranes and their associated cytoskeleton, yet remarkably little is known of how this coupling is achieved. In a GFP exon-trap screen, we identified Drosophila melanogaster Basigin (Bsg) as an immunoglobulin domain-containing transmembrane protein accumulating at periactive zones of neuromuscular junctions. Bsg is required pre- and postsynaptically to restrict synaptic bouton size, its juxtamembrane cytoplasmic residues being important for that function. Bsg controls different aspects of synaptic structure, including distribution of synaptic vesicles and organization of the presynaptic cortical actin cytoskeleton. Strikingly, bsg function is also required specifically within the presynaptic terminal to inhibit nonsynchronized evoked vesicle release. We thus propose that Bsg is part of a transsynaptic complex regulating synaptic compartmentalization and strength, and coordinating plasma membrane and cortical organization.
Figures

References
-
- Berditchevski, F., S. Chang, J. Bodorova, and M.E. Hemler. 1997. Generation of monoclonal antibodies to integrin-associated proteins. Evidence that alpha3beta1 complexes with EMMPRIN/basigin/OX47/M6. J. Biol. Chem. 272:29174–29180. - PubMed
-
- Beumer, K.J., J. Rohrbough, A. Prokop, and K. Broadie. 1999. A role for PS integrins in morphological growth and synaptic function at the postembryonic neuromuscular junction of Drosophila. Development. 126:5833–5846. - PubMed
-
- Bogdan, S., R. Stephan, C. Lobke, A. Mertens, and C. Klambt. 2005. Abi activates WASP to promote sensory organ development. Nat. Cell Biol. 7:977–984. - PubMed
-
- Bretscher, A., K. Edwards, and R.G. Fehon. 2002. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 3:586–599. - PubMed
-
- Collins, M.O., H. Husi, L. Yu, J.M. Brandon, C.N. Anderson, W.P. Blackstock, J.S. Choudhary, and S.G. Grant. 2006. Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. J. Neurochem. 97(Suppl. 1):16–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

