Nodes of Ranvier and axon initial segments are ankyrin G-dependent domains that assemble by distinct mechanisms
- PMID: 17548513
- PMCID: PMC2064285
- DOI: 10.1083/jcb.200612012
Nodes of Ranvier and axon initial segments are ankyrin G-dependent domains that assemble by distinct mechanisms
Abstract
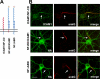
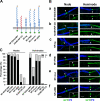
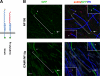
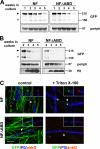
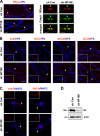
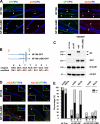
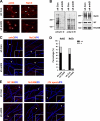
Axon initial segments (AISs) and nodes of Ranvier are sites of action potential generation and propagation, respectively. Both domains are enriched in sodium channels complexed with adhesion molecules (neurofascin [NF] 186 and NrCAM) and cytoskeletal proteins (ankyrin G and betaIV spectrin). We show that the AIS and peripheral nervous system (PNS) nodes both require ankyrin G but assemble by distinct mechanisms. The AIS is intrinsically specified; it forms independent of NF186, which is targeted to this site via intracellular interactions that require ankyrin G. In contrast, NF186 is targeted to the node, and independently cleared from the internode, by interactions of its ectodomain with myelinating Schwann cells. NF186 is critical for and initiates PNS node assembly by recruiting ankyrin G, which is required for the localization of sodium channels and the entire nodal complex. Thus, initial segments assemble from the inside out driven by the intrinsic accumulation of ankyrin G, whereas PNS nodes assemble from the outside in, specified by Schwann cells, which direct the NF186-dependent recruitment of ankyrin G.
Figures


References
-
- Ango, F., G. di Cristo, H. Higashiyama, V. Bennett, P. Wu, and Z.J. Huang. 2004. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 119:257–272. - PubMed
-
- Bennett, V., and S. Lambert. 1999. Physiological roles of axonal ankyrins in survival of premyelinated axons and localization of voltage-gated sodium channels. J. Neurocytol. 28:303–318. - PubMed
-
- Berghs, S., D. Aggujaro, R. Dirkx, E. Maksimova, P. Stabach, J.M. Hermel, J.P. Zhang, W. Philbrick, V. Slepnev, T. Ort, and M. Solimena. 2000. βIV spectrin, a new spectrin localized at axon initial segments and nodes of Ranvier in the central and peripheral nervous system. J. Cell Biol. 151:985–1002. - PMC - PubMed
-
- Boiko, T., and B. Winckler. 2003. Picket and other fences in biological membranes. Dev. Cell. 5:191–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

