Impaired accumulation of antigen-specific CD8 lymphocytes in chemokine CCL25-deficient intestinal epithelium and lamina propria
- PMID: 17548595
- PMCID: PMC2564614
- DOI: 10.4049/jimmunol.178.12.7598
Impaired accumulation of antigen-specific CD8 lymphocytes in chemokine CCL25-deficient intestinal epithelium and lamina propria
Abstract
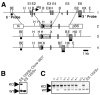
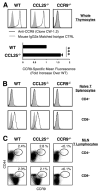
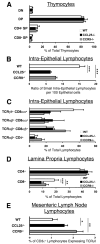
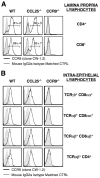
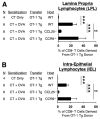
CCL25 and CCR9 constitute a chemokine/receptor pair involved in T cell development and in gut-associated immune responses. In this study, we generated CCL25(-/-) mice to answer questions that could not be addressed with existing CCR9(-/-) mice. Similar phenotypes were observed for both CCL25(-/-) and CCR9(-/-) mice, consistent with the notion that CCL25 and CCR9 interact with each other exclusively. We assessed the requirement for CCL25 in generating CCR9(high) CD8 intestinal memory-phenotype T cells and the subsequent accumulation of these cells within effector sites. TCR-transgenic naive CD8 T cells were transferred into wild-type or CCL25-deficient hosts. Oral sensitization with Ag allowed these naive donor cells to efficiently differentiate into CCR9(high) memory-phenotype cells within the mesenteric lymph nodes of wild-type hosts. This differentiation event occurred with equal efficiency in the MLN of CCL25-deficient hosts, demonstrating that CCL25 is not required to induce the CCR9(high) memory phenotype in vivo. However, we found that CCL25 deficiency severely impaired the Ag-dependent accumulation of donor-derived CD8 T cells within both lamina propria and epithelium of the small intestine. Thus, although CCL25 is not necessary for generating memory-phenotype CD8 T cells with "gut-homing" properties, this chemokine is indispensable for their trafficking to the small intestine.
Conflict of interest statement
Disclosures
The authors have no financial conflict of interest.
Figures
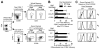


References
-
- Vicari AP, Figueroa DJ, Hedrick JA, Foster JS, Singh KP, Menon S, Copeland NG, Gilbert DJ, Jenkins NA, Bacon KB, Zlotnik A. TECK: a novel CC chemokine specifically expressed by thymic dendritic cells and potentially involved in T cell development. Immunity. 1997;7:291–301. - PubMed
-
- Wurbel MA, Philippe JM, Nguyen C, Victorero G, Freeman T, Wooding P, Miazek A, Mattei MG, Malissen M, Jordan BR, et al. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur J Immunol. 2000;30:262–271. - PubMed
-
- Zabel BA, Agace WW, Campbell JJ, Heath HM, Parent D, Roberts AI, Ebert EC, Kassam N, Qin S, Zovko M, et al. Human G protein-coupled receptor GPR-9 – 6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J Exp Med. 1999;190:1241–1256. - PMC - PubMed
-
- Benz C, Heinzel K, Bleul CC. Homing of immature thymocytes to the subcapsular microenvironment within the thymus is not an absolute requirement for T cell development. Eur J Immunol. 2004;34:3652–3663. - PubMed
-
- Uehara S, Hayes SM, Li L, El-Khoury D, Canelles M, Fowlkes BJ, Love PE. Premature expression of chemokine receptor CCR9 impairs T cell development. J Immunol. 2006;176:75– 84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

