Stochastic innovation as a mechanism by which catalysts might self-assemble into chemical reaction networks
- PMID: 17548812
- PMCID: PMC1891208
- DOI: 10.1073/pnas.0703522104
Stochastic innovation as a mechanism by which catalysts might self-assemble into chemical reaction networks
Abstract
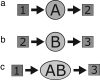
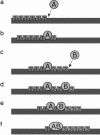
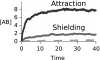
We develop a computer model for how two different chemical catalysts in solution, A and B, could be driven to form AB complexes, based on the concentration gradients of a substrate or product that they share in common. If A's product is B's substrate, B will be attracted to A, mediated by a common resource that is not otherwise plentiful in the environment. By this simple physicochemical mechanism, chemical reactions could spontaneously associate to become chained together in solution. According to the model, such catalyst self-association processes may resemble other processes of "stochastic innovation," such as Darwinian evolution in biology, that involve a search among options, a selection among those options, and then a lock-in of that selection. Like Darwinian processes, this simple chemical process exhibits cooperation, competition, innovation, and a preference for consistency. This model may be useful for understanding organizational processes in prebiotic chemistry and for developing new kinds of self-organization in chemically reacting systems.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Mayr E. What Evolution Is. New York: Basic Books; 2001.
-
- Hebb DO. The Organization of Behavior. New York: Wiley; 1949.
-
- Goda Y, Davis GW. Neuron. 2003;40:243–264. - PubMed
-
- Alberts B, Johnson A, Lewis J, Raff M., Roberts K, Walter P. Mol Biol Cell. New York: Garland Science; 2002. p. 1238.

