Dissection of the components for PIP2 activation and thermosensation in TRP channels
- PMID: 17548815
- PMCID: PMC1891241
- DOI: 10.1073/pnas.0703420104
Dissection of the components for PIP2 activation and thermosensation in TRP channels
Abstract
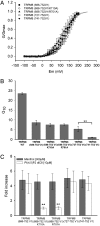
Phosphatidylinositol 4,5-bisphosphate (PIP2) plays a central role in the activation of several transient receptor potential (TRP) channels. The role of PIP2 on temperature gating of thermoTRP channels has not been explored in detail, and the process of temperature activation is largely unexplained. In this work, we have exchanged different segments of the C-terminal region between cold-sensitive (TRPM8) and heat-sensitive (TRPV1) channels, trying to understand the role of the segment in PIP2 and temperature activation. A chimera in which the proximal part of the C-terminal of TRPV1 replaces an equivalent section of TRPM8 C-terminal is activated by PIP2 and confers the phenotype of heat activation. PIP2, but not temperature sensitivity, disappears when positively charged residues contained in the exchanged region are neutralized. Shortening the exchanged segment to a length of 11 aa produces voltage-dependent and temperature-insensitive channels. Our findings suggest the existence of different activation domains for temperature, PIP2, and voltage. We provide an interpretation for channel-PIP2 interaction using a full-atom molecular model of TRPV1 and PIP2 docking analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

