Neuroprotective interventions targeting detrimental host immune responses protect mice from fatal alphavirus encephalitis
- PMID: 17549013
- PMCID: PMC3143496
- DOI: 10.1097/01.jnen.0000263867.46070.e2
Neuroprotective interventions targeting detrimental host immune responses protect mice from fatal alphavirus encephalitis
Abstract
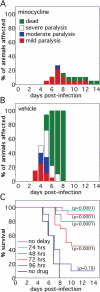
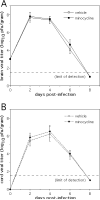
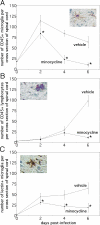
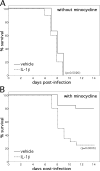
Systemic treatment with the tetracycline derivative, minocycline, attenuates neurologic deficits in animal models of amyotrophic lateral sclerosis, hypoxic-ischemic brain injury, and multiple sclerosis. Inhibition of microglial activation within the CNS is 1 mechanism proposed to underlie the beneficial effects of the drug in these systems. Given the widening scope of acute viral encephalitis caused by mosquito-borne pathogens, we investigated the therapeutic effects of minocycline in a murine model of fatal alphavirus encephalomyelitis in which widespread microglial activation is known to occur. We found that minocycline conferred significant protection against both paralysis and death, even when started after viral challenge and despite having no effect on CNS virus replication or spread. Further studies demonstrated that minocycline inhibited early virus-induced microglial activation and that diminished CNS production of the inflammatory mediator, interleukin (IL)-1beta, contributed to its protective effect. Therapeutic blockade of IL-1 receptors also conferred significant protection in our model, validating the importance of the IL-1 pathway in disease pathogenesis. We propose that interventions targeting detrimental host immune responses arising from activated microglia may be of benefit in humans with acute viral encephalitis caused by related mosquito-borne pathogens. Such treatments could conceivably act through neuroprotective rather than antiviral mechanisms to generate these clinical effects.
Figures



References
-
- Jackson AC, Moench TR, Trapp BD, et al. Basis of neurovirulence in Sindbis virus encephalomyelitis of mice. Lab Invest. 1988;58:503–509. - PubMed
-
- Jackson AC, Moench TR, Griffin DE, et al. The pathogenesis of spinal cord involvement in the encephalomyelitis of mice caused by neuroadapted Sindbis virus infection. Lab Invest. 1987;56:418–423. - PubMed
-
- Kelley TW, Prayson RA, Isada CM. Spinal cord disease in West Nile Virus infection. N Engl J Med. 2003;348:564–566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

