Adoptive T cell therapy for cancer in the clinic
- PMID: 17549249
- PMCID: PMC1878537
- DOI: 10.1172/JCI32446
Adoptive T cell therapy for cancer in the clinic
Abstract
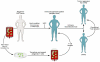
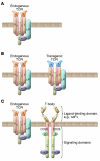
The transfusion of lymphocytes, referred to as adoptive T cell therapy, is being tested for the treatment of cancer and chronic infections. Adoptive T cell therapy has the potential to enhance antitumor immunity, augment vaccine efficacy, and limit graft-versus-host disease. This form of personalized medicine is now in various early- and late-stage clinical trials. These trials are currently testing strategies to infuse tumor-infiltrating lymphocytes, CTLs, Th cells, and Tregs. Improved molecular biology techniques have also increased enthusiasm and feasibility for testing genetically engineered T cells. The current status of the field and prospects for clinical translation are reviewed herein.
Figures

References
-
- Shankaran V., et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. - PubMed
-
- Goedert J.J. The epidemiology of acquired immunodeficiency syndrome malignancies. Semin. Oncol. 2000;27:390–401. - PubMed
-
- Southam C.M., Brunschwig A., Levin A.G., Dizon Q.S. Effect of leukocytes on transplantability of human cancer. Cancer. 1966;19:1743–1753. - PubMed
-
- Zhang L., et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. . N. Engl. J. Med. 2003;348:203–213. - PubMed
-
- Galon J., et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

