Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology
- PMID: 17549256
- PMCID: PMC1878531
- DOI: 10.1172/JCI31450
Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology
Abstract
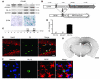
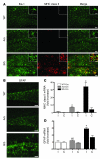
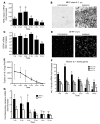
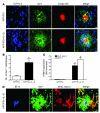
Neuroinflammation is a conspicuous feature of Alzheimer disease (AD) pathology and is thought to contribute to the ultimate neurodegeneration that ensues. IL-1 beta has emerged as a prime candidate underlying this response. Here we describe a transgenic mouse model of sustained IL-1 beta overexpression that was capable of driving robust neuroinflammation lasting months after transgene activation. This response was characterized by astrocytic and microglial activation in addition to induction of proinflammatory cytokines. Surprisingly, when triggered in the hippocampus of the APPswe/PS1dE9 mouse model of AD, 4 weeks of IL-1 beta overexpression led to a reduction in amyloid pathology. Congophilic plaque area fraction and frequency as well as insoluble amyloid beta 40 (A beta 40) and A beta 42 decreased significantly. These results demonstrate a possible adaptive role for IL-1 beta-driven neuroinflammation in AD and may help explain recent failures of antiinflammatory therapeutics for this disease.
Figures


Comment in
-
A beneficial role for IL-1 beta in Alzheimer disease?J Clin Invest. 2007 Jun;117(6):1483-5. doi: 10.1172/JCI32356. J Clin Invest. 2007. PMID: 17549252 Free PMC article.
References
-
- Basu A., Krady J.K., Levison S.W. Interleukin-1: a master regulator of neuroinflammation. J. Neurosci. Res. 2004;78:151–156. - PubMed
-
- Allan S.M., Tyrrell P.J., Rothwell N.J. Interleukin-1 and neuronal injury. Nat. Rev. Immunol. 2005;5:629–640. - PubMed
-
- Van Everbroeck B., et al. The role of cytokines, astrocytes, microglia and apoptosis in Creutzfeldt-Jakob disease. Neurobiol. Aging. 2002;23:59–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

